Byron's Bender
|
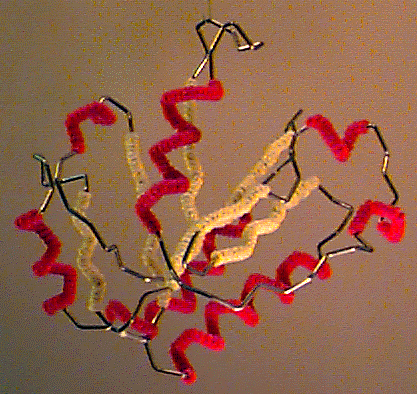
Byron's Bender wire model of the backbone trace of the I domain of CD11a (from 1lfa), an alpha/beta domain of a leukocyte integrin adhesion molecule. Alpha helices are decorated with red pipe cleaners, while beta strands are decorated yellow. Model generously constructed by Tim Herman in 1997. |
Byron's Bender, invented by crystallographer Byron Rubin[1][2], is a machine designed to bend wire into the backbone trace of a protein model. The machine was manufactured by the Charles Supper Company in Natick Massachusetts[3], and widely used by crystallographers in the 1970's and 1980's. Photos of the machine are available[1][2][3][4].
Wire is advanced in fixed-length increments, representing the distances between alpha-carbon atoms. A dihedral angle is then set on dials, and a lever is pulled to bend the wire to the specified angle. Often, the emerging wire model collides with the machine. Then, the wire is cut, and later the resulting segments are fastened end to end with small metal sleeves. Each such joint is silver-soldered or glued at the correct angle.
See AlsoSee Also
Notes & ReferencesNotes & References
- ↑ 1.0 1.1 Rubin, Byron; Richardson Jane S. The simple construction of protein alpha-carbon models. Biopolymers. 1972; 11(11):2381-5. PDF
- ↑ 2.0 2.1 Rubin, Byron. 1985. Macromolecule backbone models. Methods in Enzymology 115:391-7.
- ↑ 3.0 3.1 Supper Protein Wire Model Bender
- ↑ 1970 - Byron's Bender by Pierre Dragicevic, 2015.