4n3z
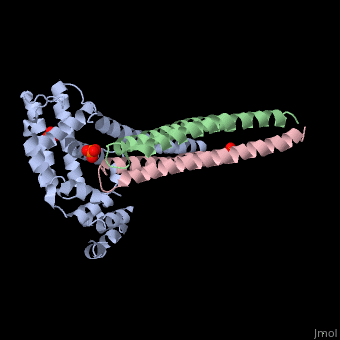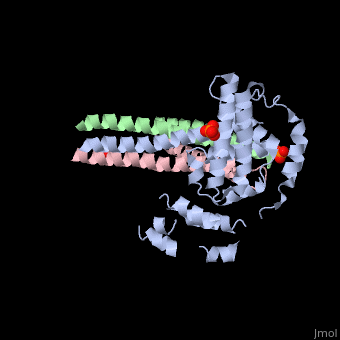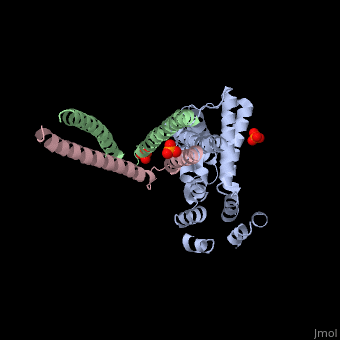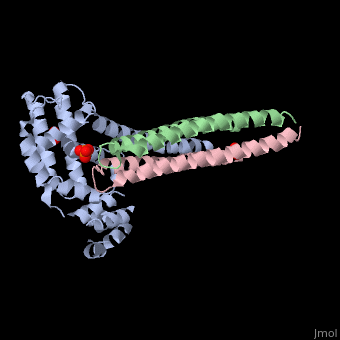
Crystal structure of Rabex-5delta and Rabaptin-5C21 complexCrystal structure of Rabex-5delta and Rabaptin-5C21 complex
Structural highlights
Function[RABX5_HUMAN] Rab effector protein acting as linker between gamma-adaptin, RAB4A or RAB5A. Involved in endocytic membrane fusion and membrane trafficking of recycling endosomes. Stimulates nucleotide exchange on RAB5A. Can act as a ubiquitin ligase (By similarity).[1] [2] [3] [RABE1_HUMAN] Rab effector protein acting as linker between gamma-adaptin, RAB4A and RAB5A. Involved in endocytic membrane fusion and membrane trafficking of recycling endosomes. Stimulates RABGEF1 mediated nucleotide exchange on RAB5A.[4] [5] [6] [7] Publication Abstract from PubMedRabex-5 and Rabaptin-5 function together to activate Rab5 and further promote early endosomal fusion in endocytosis. The Rabex-5 GEF activity is autoinhibited by the Rabex-5 CC domain (Rabex-5CC) and activated by the Rabaptin-5 C2-1 domain (Rabaptin-5C21) with yet unknown mechanism. We report here the crystal structures of Rabex-5 in complex with the dimeric Rabaptin-5C21 (Rabaptin-5C212) and in complex with Rabaptin-5C212 and Rab5, along with biophysical and biochemical analyses. We show that Rabex-5CC assumes an amphipathic alpha-helix which binds weakly to the substrate-binding site of the GEF domain, leading to weak autoinhibition of the GEF activity. Binding of Rabaptin-5C21 to Rabex-5 displaces Rabex-5CC to yield a largely exposed substrate-binding site, leading to release of the GEF activity. In the ternary complex the substrate-binding site of Rabex-5 is completely exposed to bind and activate Rab5. Our results reveal the molecular mechanism for the regulation of the Rabex-5 GEF activity. Molecular mechanism for Rabex-5 GEF activation by Rabaptin-5.,Zhang Z, Zhang T, Wang S, Gong Z, Tang C, Chen J, Ding J Elife (Cambridge). 2014 Jun 23:e02687. doi: 10.7554/eLife.02687. PMID:24957337[8] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. References
|
| ||||||||||||||||||||