IGF1
Insulin-like growth factor 1Insulin-like growth factor 1
Insulin is a very important endocrine protein. Indeed, it is the only hypoglycemic hormone of the human body. This protein is secreted in the beta cells of the Langerhans’ islet in the pancreas and takes part in the glycogenesis. This molecule helps the transportation of glucose into the cells, thus reducing the blood sugar rate, contrary to glucagon. Insulin also has a huge effect on growth and thereby has a lot of structural similarities with its main associated growth factor: IGF-1, aka somatomedin. Both share lots of properties thanks to their 60% similarity. IGF-1 is a peptidic hormone of 69 amino acids which is secreted by the liver. This protein deals with a large scale of regulations, from growth to nutrition and it is even implied in stress response, breeding and longevity.[1]. It is the main actor in primary growth cell control.
HistoryThe role of the pancreas and therefore insulin in diabete was first discovered by Oskar von Minkowski in Strasbourg in 1889. IGF1 was first identified in 1957 by the team of Salmon and Daughaday. In 1976, Rinderknecht and Humbel isolated IGF1 from human cells. The protein was further analysed in 1987 by the team of Dr Mike Davis (Imperial College of London, United Kingdom) and Pr Robin Jokin (INSERM of Toulouse).
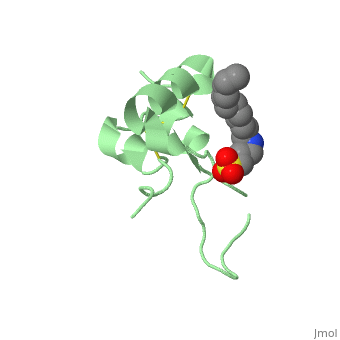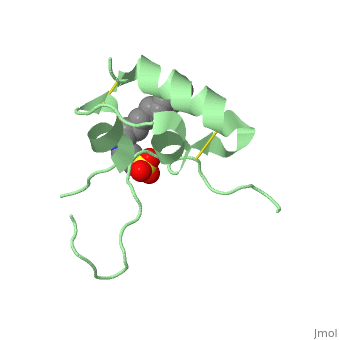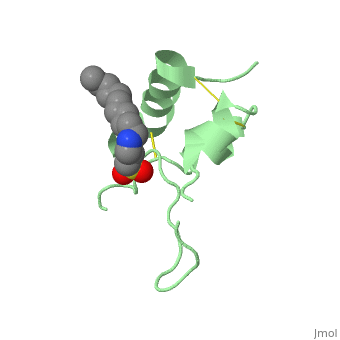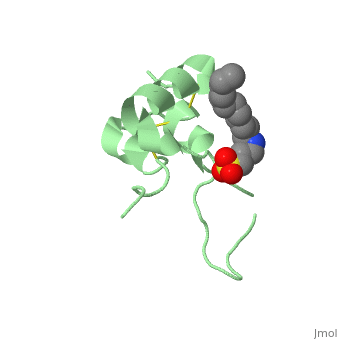
Biological structures and interactionsIn the pituitary gland inside the brain, Growth Hormone (GH) is secreted and its release enable transcription of IGF-1 in liver and depending on the nutritional state, a paracrine or autocrine activation IGF-1 occurs. IGF-1 then acts as a ligand and can interact with Insulin Receptor protein and Insulin-like Growth Factor Binding Protein. StructuresIGF-1 is a 8.28 kDa protein consisting of 69 amino acids. The coding RNA of this protein creates 4 isoforms differing from each other due to RNA splicing. Nevertheless, all 4 of them have cysteine residues that are able to make disulfide bond. This enable them to link to IGF-1 receptor. Its secondary structure as shown here is composed of alpha helix and beta strand. Its 3D structure is shown on the right. Stimulating interaction : IGF1 - IGF1RIGF1R is a transmembrane protein receptor. It is composed of two alpha subunits and two tyrosine beta subunits. Both alpha subunits are cysteine-rich region and therefore linked with a disulfide bond. Ligand-binding on alpha subunit induces activation of beta subunit by autophosphorylation. It further leads to activation of the Akt and mTor pathways inside the cell. Due to their homology sequences, the three members of the insulin protein family: IGF1, IGF2 and Insulin can interact with one another of the different receptors. Hence, Insulin binds to IGF1R following the same mechanism and activated the intracellular pathways in the same way IGF1 does. Therefore, the various concentration of the insulin proteins regulates the cell activity in different context, for instance in excess of glucose or lack of Growth Hormone.
Inhibiting interaction : IGF1 - IGFBPIGF binding proteins (IGFBPs) weight 24 to 45 kDa. All six IGFBPs share 50% homology with each other and have binding affinities at the same order of magnitude for IGF-I and IGF-II but have greater affinities then IGF-1 for its receptor. Once IGF1 is bound to Insulin-like Growth Binding Protein (IGFBP), IGF1 cannot be linked to IGF1R any longer. Therefore, increases in serum levels of this IGFBP result in a decrease of IGF-1 activity thus inhibiting the cellular pathways. The IGFBPs help to lengthen the half-life of circulating IGFs in all tissues. That is why approximately 98% of IGF-1 exists as complexed form with one of the six different IGFBP. IGFBP-3, the most abundant protein, accounts for 80% of all IGF binding. Inside the liver, this mechanism is responsible for positive feedback, more precisely it allows growth hormone to continuously act upon the liver to produce more IGF-1.
MetabolismBiological contextSecretion of GH induces IGF1 transcription but IGF1 can also be secreted by the liver without GH stimulation. After binding to its receptors (IGF1R and insulin receptor), IGF1 activates a signalling cascade by autocrine or paracrine way resulting in cell growth, proliferation and survival against apoptose. However IGFBPs can activate or inhibate IGF1 actions on their target cells.[2] [3] If IGF1 is lacking, it reduces cell growth and can also induce dysfunctions or even apoptose of cells, thus creating diseases. Related diseasesGrowth failure, diabetes, cardiovascular pathology and others diseases such as Laron syndrom or cancers can be the consequences of an IGF1 low level. Knowing that IGF1 leads to cell proliferation with apoptose inhibition, if DNA undergoes alterations or mutations, those alterated cells will proliferate and survive even though it should result in their elimination by apoptose. Hence inducing cancer proliferation. Cite error: Invalid The Laron syndrom, caused by mutation of GH genes, features growth failure and high insulin sensibility. Thus reducing concentration of GH receptors and inducing an IGF1 low level. Treatment is based on biosynthetic IGF1 carry out in mecasermin (a drug developed by Genentech) with IGFBP3 and recombinant human IGF1. One main side effect is hypoglycemia due to IGF1 activity. Cite error: Invalid ApplicationsClinical trials with IGF1 injection on persons affected by diabetes type I and II, brought to light an improvement of glycemic control and a reduction of insulin injection necessarly to keep a normal glycemic regulation. But due to side effects such as pain, this treatment was interrupted until recently, with the introduction of an IGF1 and IGFBP3 combination, clinical trials seemed to display the same results as previously, nevertheless with reduced side effects. Cite error: Invalid Other clinical trials have shown higher IGF1 levels for patients with Parkinson advanced diseases, it doesn’t occur clear enough at early stages, compared to healthy persons. Nevertheless, the relationship between IGF1 levels and Parkinson disease duration or severity stays uncertain. That is why this trial concludes to an IGF1 limited prediction marker in Parkinson disease even if it could be a biomarker most pronounced in advanced stage disease. Cite error: Invalid On the contrary, a lower IGF1 is associated with an increased risk to develop an Alzheimer disease. According to this other trial, IGF1 higher levels may protect against subclinical and clinical neurodegeneration.Cite error: Invalid
|
| ||||||||||
ReferencesReferences
- ↑ Patrick Jouandin. Rôle de la voie de signalisation Insuline dans le couplage des informations nutritionnelles et développementales au cours de l'ovogenèse chez la drosophile. Sciences agricoles.Université Nice Sophia Antipolis, 2013. Français.<NNT : 2013NICE4102>.<tel-00932409>
- ↑ http://www.hal.inserm.fr/tel-00932409/,26/01/2017
- ↑ Elena Conti, Maria Beatrice Musumeci, et all, IGF-1 and atherothrombosis: relevance to pathophysiology and therapy,Clinical Science May 01, 2011, 120 (9) 377-402