Sandbox 45673
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamideN-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide
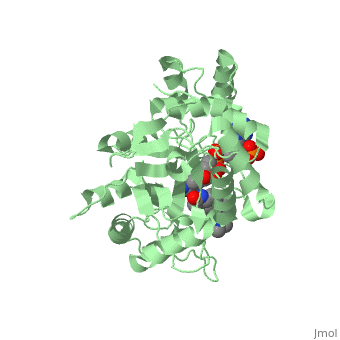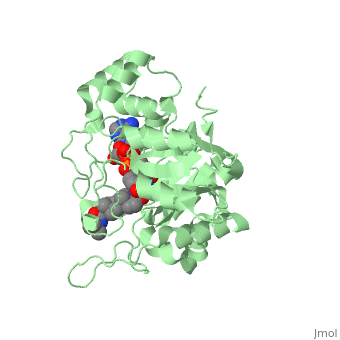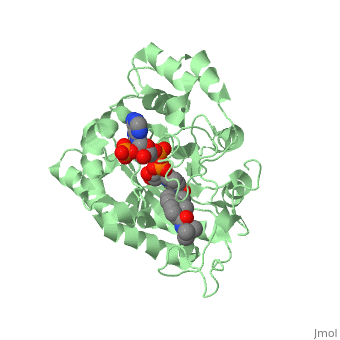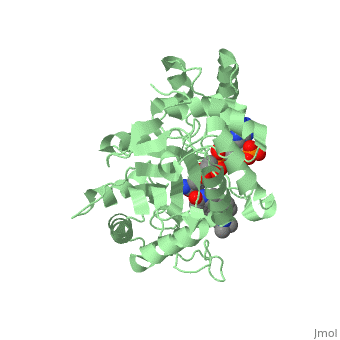
FunctionFinasteride is a synthetic 4-azasteroid compound that acts as a 5α-reductase inhibitor.[1] The 5α-reductase enzyme is very important in the metabolism of many of the steroids produced by the body, in particular the conversion of testosterone to dihydrotestosterone (DHT).[1] For this reason, Finasteride is used as a treatment for benign prostate hyperplasia (BPH), which is caused by an overproduction of DHT in the male prostate.[2] Pattern hair loss, another condition in men caused by the build up of DHT, can also be treated with Finasteride.[3] Finasteride's affinity for 5α-reductase approaches that of testosterone, and can bind to two of the three isoenzymes of 5α-reductase, types I and II.[2] StructureTo see what Finasteride looks like unbound, click this link: . Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together [4]. Although gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer [5]. The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids. The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a tert-butyl group bonded to the nitrogen atom, has been added onto the carbon 17. MechanismFinasteride is a 5-alpha reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.[6]Typically 5α-redcutase turns testosterone into Dihydrotestosterone(DHT), but the enzyme will accept Finasteride as an alternate substrate; turning it into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct. Finasteride is similar in structure to testosterone and 5alpha-reductase has almost the same affinity for both molecules. However, Finasteride , having a high affinity for 5α-reductase, covalently binds to the enzyme as a Michael acceptor, through a functionally irreversible reaction. However, the NADP-dihydrofinasteride complex breaks down with a half life of about 1 month at 37˚C., which is why patients must continue taking the drug.[7]  Medical UsesFinasteride is used to shrink an enlarged prostate, also known as benign prostatic hyperplasia (BPH), in adult men (Allen, 2015). Enlarged prostate is located near the bladder which causes difficulty with passing urine. Common symptoms include, long period of time before urine flow, feeling the bladder is not empty, and dribbling urine (Allen, 2015). This medication works by blocking the enzyme 5α-reductase, which prevents conversion of testosterone to the natural body hormone, dihydrosestosterone (DHT) that causes growth of the prostate. As a result, reducing the amount of dihydrotestosterone causes the prostate to shrink. Thus, helps the urine pass easily. *I plan to add hyperlinks* Finasteride can also lead to improvements in Androgenetic alopecia, male pattern baldness (MPB), which is caused by an androgen-dependent miniaturization of scalp hair follicles. Testosterone is the major flow of androgen, but to be maximally active in scalp hair follicles it must be converted to dihydrotestosterone (DHT) by the enzyme 5a- reductase. DHT is an important factor in MPB by the absence of the condition in males with a insufficiency of type II 5a-reductase, and by small amounts of hair regrowth in men with MPB (Olsen, et al., 2006). Finasteride acts as an inhibitor for the type II 5a-reductase enzyme, which has shown to reduce both serum and scalp skin dihydrotestosterone levels in balding men (Leyden, et al., 1999). Side effects from Finasteride include but are not limited to, decreased sexual ability and desire.
To do list1.) Hyperlink to other articles 2.) Go into more depth with each section, especially structure and function 3.) Add synthesis
|
| ||||||||||
ReferencesReferences
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t Cite error: Invalid
<ref>tag; name "five" defined multiple times with different content
Allen, Helen. (2015, March). "Finasteride for prostate gland enlargement. Information. Patient.
Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
Leyden, J., Dunlap, F., & Miller, B., et el. (1999, June). Finasteride in the treatment of men with frontal male pattern hair loss.
Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm