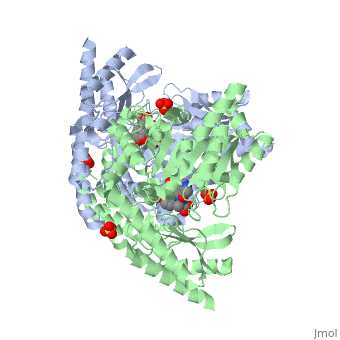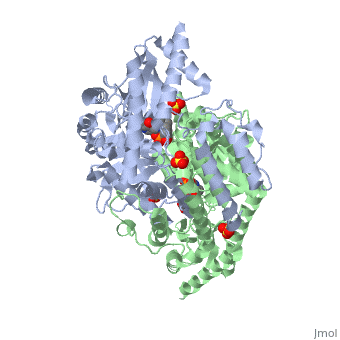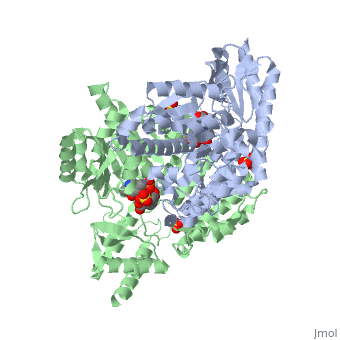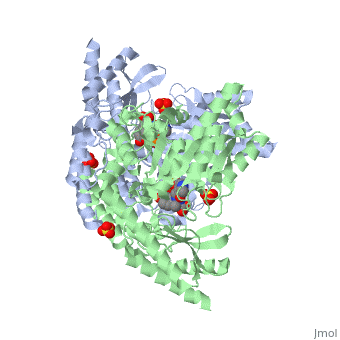
Carbidopa
FunctionFunction
(Lodosyn) is a drug given, in conjunction with Levadopa, to those with Parkinson's Disease in order to inhibit the peripheral metabolism of Levadopa, allowing a greater portion of the drug to cross the blood-brain barrier and produce the desired effect on the central nervous system. It works by inhibiting the enzymatic activity of aromatic-L-amino-acid decarboxylase (DOPA Decarboxylase or DDC)[1] StructureCarbidopa is an inhibitor of DDC. It is a partially water soluble, white, crystalline compound with a molecular weight of 226.232g/mol. Its empirical formula is C10H14N2O4•H2O and its IUPAC name is (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid. Used in conjunction with Levadopa (L-DOPA), a precursor to dopamine, it increases concentrations of L-DOPA in the brain. Due to the fact Carbidopa cannot cross the blood–brain barrier, it inhibits only peripheral DDC thus preventing the conversion of L-DOPA to dopamine outside of neuronal cells. This greatly lessens the side effects caused by dopamine on the periphery, as well as increasing the concentration of L-DOPA and subsequently dopamine in the brain. Molecular MechanismThe conversion of L-DOPA into dopamine is catalyzed by the vitamin B6 ()-dependent enzyme DDC, an enzyme abundant in the nervous system as well as kidneys of humans[2] The catalytically active form of DDC is a homodimer, a feature typical of this class of enzymes[3]. DDCs active site is located between the two monomers but is composed mainly of residues from only one of the monomers. The cofactor PLP binds to Lys 303 through a Schiff base linkage and a salt bridge between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP, which is able to provide a strong electron sink capable of stabilizing the carbanionic intermediates produced by active DDC. Carbidopa works by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety and blocking the DDC residues Ile 101' and Phe 103' in the substrate binding pocket with its catechol ring[4]. Due to the fact that Carbidopa cannot cross the blood-brain barrier, its inhibiting effects only are displayed in the periphery. Disease in HumansParkinson's disease (PD) is a chronic, progressive neurological disease whose symptoms include bradykinesia, tremors, postural instability and rigidity. Although the exact cause of the disease is currently unknown, it is believe to be caused by the apoptosis of dopanergic cells in the substantia nigra of the brain and subsequent loss of dopamine.[5]
|
| ||||||||||
ReferencesReferences
- ↑ Gilbert JA, Frederick LM, Ames MM. The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res. 2000 Nov;6(11):4365-72. PMID:11106255
- ↑ Opacka-Juffry J, Brooks DJ. L-dihydroxyphenylalanine and its decarboxylase: new ideas on their neuroregulatory roles. Mov Disord. 1995 May;10(3):241-9. PMID:7651438 doi:http://dx.doi.org/10.1002/mds.870100302
- ↑ Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000 Jan 15;8(1):R1-6. PMID:10673430
- ↑ Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
- ↑ Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000 Mar 23;404(6776):394-8. PMID:10746727 doi:http://dx.doi.org/10.1038/35006074