Sandbox Reserved 439
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Asp Receptor Ligand-binding domain (1wat)[1]Asp Receptor Ligand-binding domain (1wat)[1]
This is an example page. Edit appropriately for your project.
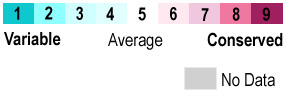
by [list all teammate names here] Student Projects for UMass Chemistry 423 Spring 2016 Overall StructureThe ligand binding domain of the aspartate receptor () ) is a dimer of two 4-helix bundles that is shown here with the bound.[2] In this the N and C termini are at the bottom of the structure; this is where the connections to the transmembrane helices have been truncated. Binding InteractionsWhen the protein is colored according to , residues at the ligand site are the most conserved. Interactions that stabilize ligand binding[3] include hydrogen bonding from Tyr149 and Gln152 backbone carbonyls and Thr154 sidechain OH to the and hydrogen bonding from the sidechain nitrogens of Arg64, Arg69, and Arg73 to the two . Additional FeaturesQuiz Question 1See AlsoCreditsIntroduction - name of team member Overall Structure - name of team member Binding Interactions - name of team member Additional Features - name of team member Quiz Question 1 - name of team member References
|
| ||||||||||