4urt
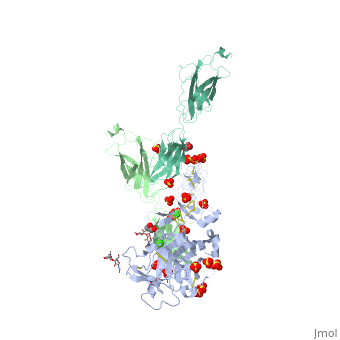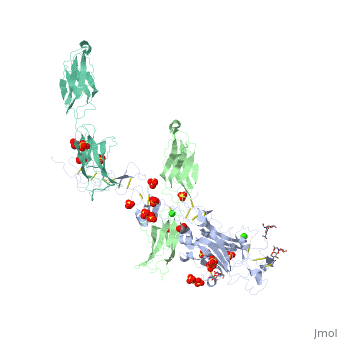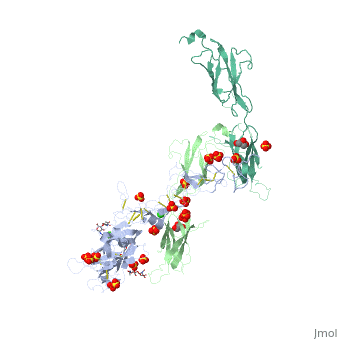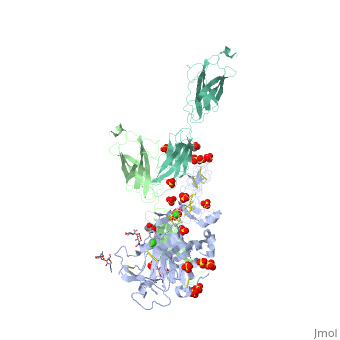
The crystal structure of a fragment of netrin-1 in complex with FN5- FN6 of DCCThe crystal structure of a fragment of netrin-1 in complex with FN5- FN6 of DCC
Structural highlights
Disease[DCC_HUMAN] Defects in DCC are the cause of mirror movements type 1 (MRMV1) [MIM:157600]. A disorder characterized by contralateral involuntary movements that mirror voluntary ones. While mirror movements are occasionally found in young children, persistence beyond the age of 10 is abnormal. Mirror movements occur more commonly in the upper extremities.[1] Function[NET1_HUMAN] Netrins control guidance of CNS commissural axons and peripheral motor axons. Its association with either DCC or some UNC5 receptors will lead to axon attraction or repulsion, respectively. It also serve as a survival factor via its association with its receptors which prevent the initiation of apoptosis. Involved in tumorigenesis by regulating apoptosis.[2] [DCC_HUMAN] Receptor for netrin required for axon guidance. Mediates axon attraction of neuronal growth cones in the developing nervous system upon ligand binding. Its association with UNC5 proteins may trigger signaling for axon repulsion. It also acts as a dependence receptor required for apoptosis induction when not associated with netrin ligand. Implicated as a tumor suppressor gene.[3] [4] Publication Abstract from PubMedNetrin-1 is a guidance cue that can trigger either attraction or repulsion effects on migrating axons of neurons, depending on the repertoire of receptors available on the growth cone. How a single chemotropic molecule can act in such contradictory ways has long been a puzzle at the molecular level. Here we present the crystal structure of netrin-1 in complex with the Deleted in Colorectal Cancer (DCC) receptor. We show that one netrin-1 molecule can simultaneously bind to two DCC molecules through a DCC-specific site and through a unique generic receptor binding site, where sulfate ions staple together positively charged patches on both DCC and netrin-1. Furthermore, we demonstrate that UNC5A can replace DCC on the generic receptor binding site to switch the response from attraction to repulsion. We propose that the modularity of binding allows for the association of other netrin receptors at the generic binding site, eliciting alternative turning responses. The Crystal Structure of Netrin-1 in Complex with DCC Reveals the Bifunctionality of Netrin-1 As a Guidance Cue.,Finci LI, Kruger N, Sun X, Zhang J, Chegkazi M, Wu Y, Schenk G, Mertens HD, Svergun DI, Zhang Y, Wang JH, Meijers R Neuron. 2014 Aug 20;83(4):839-49. doi: 10.1016/j.neuron.2014.07.010. Epub 2014, Aug 7. PMID:25123307[5] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||