Gunnar Reiske/Sandbox 102
How Gluten Protein Structure Stimulates an Immune ResponseHow Gluten Protein Structure Stimulates an Immune Response
IntroductionIntroduction
The protein, gluten is found in wheat and grains such as rye and barley. Gluten is also involved with inducing an inflammatory response in individuals with celiac disease. Individuals who have the disease cannot digest gluten due to the protein’s structure, which will damage the small intestine. In detail, if an individual with celiac disease ingests foods containing gluten, the immune system responds by damaging the villi, which are fingerlike projections lining the small intestine. This type of immune response denies the body’s ability to absorb nutrients that pass through the small intestine and into the bloodstream. As a result of the damaged villi, people with celiac disease can become malnourished. Although celiac disease is genetic, the question of how the protein triggers an immune response in the gastrointestinal tract of affected individuals was further explored.
Gluten is a protein complex comprised of gliadin and glutenin. Gliadins, for those with celiac disease, are the principle toxic component of gluten and are composed of proline and glutamine peptide sequences. The peptides enter the circulatory system and come into contact with lymphocytes and T cells, resulting in the release of inflammatory chemicals. The inflammatory chemicals interact with the villi of the small intestine and damage them, disabling the body from nutrient absorption. The symptoms can include abdominal pain, weight loss, fatigue, and many other symptoms associated with malnutrition. As of now, the only treatment for celiac disease is the total exclusion of gluten from the person’s diet.[1]
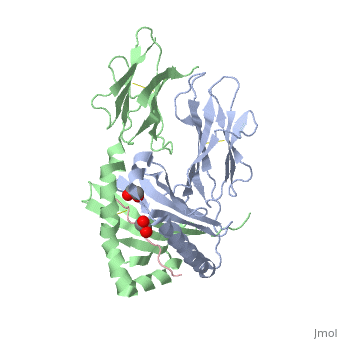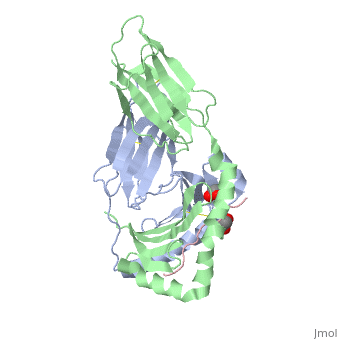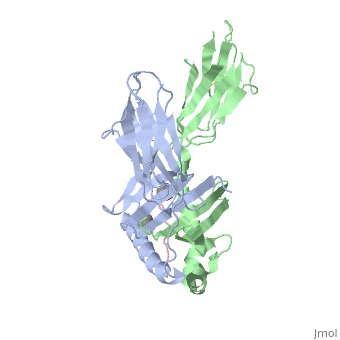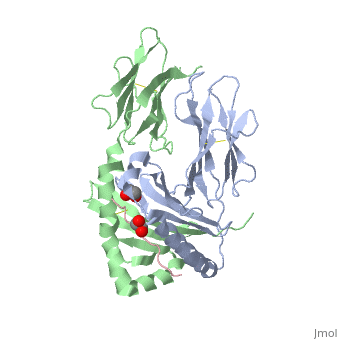
Gluten Protein ComplexFunctionThe gluten protein complex is made up of gliadin and glutenin components. Of the complex, gliadin directly affects the induction of an innate immune response via the proline and glutamine peptide sequences. In the small intestine of patients with celiac disease, HLA-DQ2 restricted T-cells are present. After ingestion of a gluten product, the gliadin peptides enter the circulatory system and come into contact with lymphocytes and the gliadin-specific, HLA-DQ2 restricted T-cells, which is the fundamental step in producing the inflammatory response associated with celiac disease.[2] RelevanceHuman leukocyte antigens (HLA) are responsible for regulation of the immune system. The binding of gliadin peptides to HLA should be the same in celiac and non-celiac patients. However, it is unclear why only specific individuals produce the gliadin-specific, HLA-DQ2 restricted T-cells with pathogenic consequences. These implications suggest an underlying genetic component. [3] Immune ResponseHLA-DQ2 and HLA-DQ8InteractionsThe HLA-DQ2 protein complex binds to the gluten complex through the use of hydrogen bonds via oxygen and nitrogen. The proline amino acids are all exposed to the external environment and do not participate in hydrogen binding. Only proline rich complexes are able to hydrogen bind properly with HLA-DQ2, such as gliadin. All other proteins do not have the proper conformation and organization of proline to effectively bind HLA-DQ2.[4] When the gluten peptide enters the HLA-DQ8 antigen binding cleft and makes contact, numerous contacts are made.[5] Notable contacts include: 16 direct hydrogen bonds, 24 water mediated hydrogen bonds, and four salt bridges. The side chains of two glutamic acids and one phenylalanine buried deep in pockets of the complex serve to anchor the peptide within the antigen-binding groove. A proline and serine can be found in shallow pockets with their side chain serving as minor anchor residues to the peptide. Treatments1yr2(placeholder)2bkl(placeholder)Structural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ Celiac Disease: MedlinePlus. Retrieved October 27, 2015, from https://www.nlm.nih.gov/medlineplus/celiacdisease.html
- ↑ Luigi Maiuri, Carolina Ciacci, Ida Ricciardelli, Loredana Vacca, Valeria Raia, Salvatore Auricchio, Jean Picard, Mohamed Osman, Sonia Quaratino, Marco Londei, Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease, The Lancet, Volume 362, Issue 9377, 5 July 2003, Pages 30-37, ISSN 0140-6736, http://dx.doi.org/10.1016/S0140-6736(03)13803-2. (http://www.sciencedirect.com/science/article/pii/S0140673603138032)
- ↑ Luigi Maiuri, Carolina Ciacci, Ida Ricciardelli, Loredana Vacca, Valeria Raia, Salvatore Auricchio, Jean Picard, Mohamed Osman, Sonia Quaratino, Marco Londei, Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease, The Lancet, Volume 362, Issue 9377, 5 July 2003, Pages 30-37, ISSN 0140-6736, http://dx.doi.org/10.1016/S0140-6736(03)13803-2. (http://www.sciencedirect.com/science/article/pii/S0140673603138032)
- ↑ Kim, Quartsen, Bergsen, Khosla, & Sollid. (n.d.). Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Cross Mark, 101(12), 4175-4179. March 2004 http://www.pnas.org/content/101/12/4175.figures-only
- ↑ Kate N. Henderson, Jason A. Tye-Din, Hugh H. Reid, Zhenjun Chen, Natalie A. Borg, Tim Beissbarth, Arthur Tatham, Stuart I. Mannering, Anthony W. Purcell, Nadine L. Dudek, David A. van Heel, James McCluskey, Jamie Rossjohn, Robert P. Anderson, A Structural and Immunological Basis for the Role of Human Leukocyte Antigen DQ8 in Celiac Disease, Immunity, Volume 27, Issue 1, 27 July 2007, Pages 23-34, ISSN 1074-7613, http://dx.doi.org/10.1016/j.immuni.2007.05.015. (http://www.sciencedirect.com/science/article/pii/S1074761307003275)