Gunnar Reiske/Sandbox 102
How Gluten Protein Structure Stimulates an Immune ResponseHow Gluten Protein Structure Stimulates an Immune Response
IntroductionIntroduction
The protein, gluten is found in wheat and grains such as rye and barley. Gluten is also involved with inducing an inflammatory response in individuals with celiac disease. Individuals who have the disease cannot digest gluten due to the protein’s structure, which will damage the small intestine. In detail, if an individual with celiac disease ingests foods containing gluten, the immune system responds by damaging the villi, which are fingerlike projections lining the small intestine. This type of immune response denies the body’s ability to absorb nutrients that pass through the small intestine and into the bloodstream. As a result of the damaged villi, people with celiac disease can become malnourished. Although celiac disease is genetic, the question of how the protein triggers an immune response in the gastrointestinal tract of affected individuals was further explored.
Gluten is a protein complex comprised of gliadin and glutenin. Gliadins, for those with celiac disease, are the principle toxic component of gluten and are composed of proline and glutamine peptide sequences. The peptides enter the circulatory system and come into contact with lymphocytes and T cells, resulting in the release of inflammatory chemicals. The inflammatory chemicals interact with the villi of the small intestine and damage them, disabling the body from nutrient absorption. The symptoms can include abdominal pain, weight loss, fatigue, and many other symptoms associated with malnutrition. As of now, the only treatment for celiac disease is the total exclusion of gluten from the person’s diet.
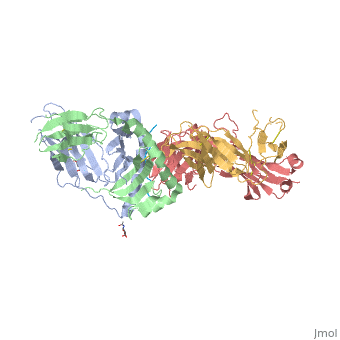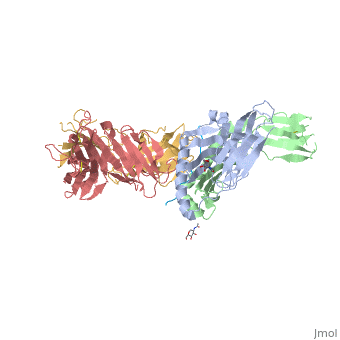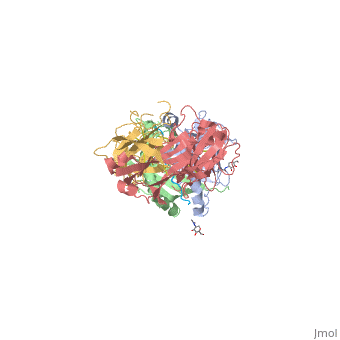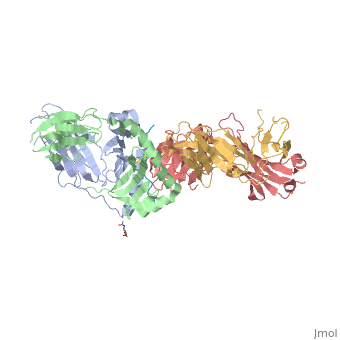
Gluten Protein ComplexFunctionThe gluten protein complex is made up of gliadin and glutenin components. Of the complex, gliadin directly affects the induction of an innate immune response via the proline and glutamine peptide sequences. In the small intestine of patients with celiac disease, HLA DQ2 restricted T-cells are present. After ingestion, the gliadin peptides enter the circulatory system and come into contact with lymphocytes and the gliadin-specific, HLA DQ2 restricted T-cells, which is the fundamental step in producing an inflammatory response associated with celiac disease (Maiuri, 2003). RelevanceIt is unclear why only specific indiviudals produce this pathogenic and T-cell specific activation. Biochemically, HLA binding of gliadin peptides should be the same in celiac and non-celiac patients. These implications suggest an underlying genetic component (Maiuri, 2003). DiseaseHLA-DQ2HLA-DQ8Background When gliadin of the gluten complex makes contact with the intestinal lumen, specifically the transglutaminase tissue, the complex HLA-DQ8 is formed[1]. This complex is comprised of three chains, two being MHC class II antigens of alpha helical and beta sheet nature with the third being the gluten peptide. The MHC class II molecule HLA-DQ8 is one of the two human leukocyte antigens associated the genetic risk of developing celiac disease and serves as a MHC class II molecule in the immune system of the body. The molecular basis of how HLA-DQ8 causes celiac disease differs from that of HLA-DQ2, however the underlying mechanisms of how it causes celiac disease are not well understood. Interactions When the gluten peptide enters the HLA-DQ8 antigen binding cleft and makes contact, numerous contacts are made[2]. Notable contacts include: 16 direct hydrogen bonds, 24 water mediated hydrogen bonds, and four salt bridges. The side chains of two glutamic acids and one phenylalanine buried deep in pockets of the complex serve to anchor the peptide within the antigen-binding groove. A proline and serine can be found in shallow pockets with their side chain serving as minor anchor residues to the peptide. Treatments1yr2(placeholder)2bkl(placeholder)Structural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ CECILIO, Lucila Arantes, & BONATTO, Mauro W.. (2015). THE PREVALENCE OF HLA DQ2 AND DQ8 IN PATIENTS WITH CELIAC DISEASE, IN FAMILY AND IN GENERAL POPULATION. ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo), 28(3), 183-185. https://dx.doi.org/10.1590/S0102-67202015000300009
- ↑ Kate N. Henderson, Jason A. Tye-Din, Hugh H. Reid, Zhenjun Chen, Natalie A. Borg, Tim Beissbarth, Arthur Tatham, Stuart I. Mannering, Anthony W. Purcell, Nadine L. Dudek, David A. van Heel, James McCluskey, Jamie Rossjohn, Robert P. Anderson, A Structural and Immunological Basis for the Role of Human Leukocyte Antigen DQ8 in Celiac Disease, Immunity, Volume 27, Issue 1, 27 July 2007, Pages 23-34, ISSN 1074-7613, http://dx.doi.org/10.1016/j.immuni.2007.05.015. (http://www.sciencedirect.com/science/article/pii/S1074761307003275)