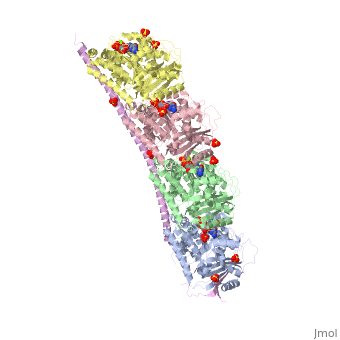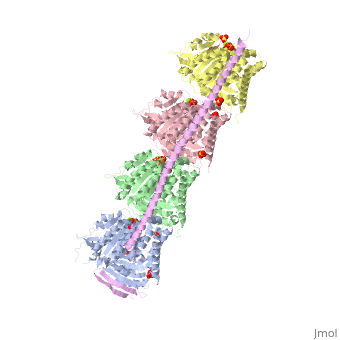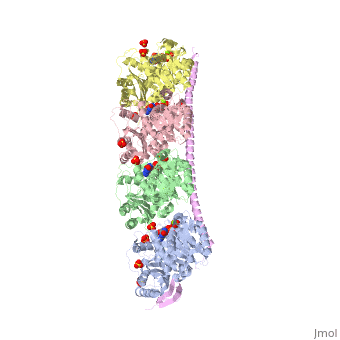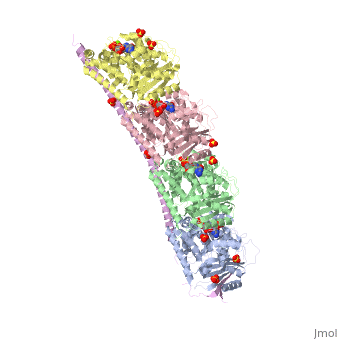
Stathmin
Stathmin (STM) regulates microtubules dynamics. Microtubules undergo continuous assembly and disassembly in the cell’s cytoskeleton. STM binds to tubulin and prevents the latter from polymerization thus preventing microtubule assembly. Phosphorylation of STM weakens the binding of STM to tubulin enabling the microtubule assembly needed for the formation of mitotic spindle. Thus, STM is an oncoprotein. STM contains an SLD (Stathmin-Like Domain) domain of 149 residues which binds the tubulin dimer. FunctionDiseaseRelevanceStructural highlights |
| ||||||||||
3D Structures of stathmin3D Structures of stathmin
Updated on 20-July-2015