Sandbox Reserved 992
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
Beta-lactamases are awesome
|
Background and beta-lactam antibioticsBackground and beta-lactam antibiotics
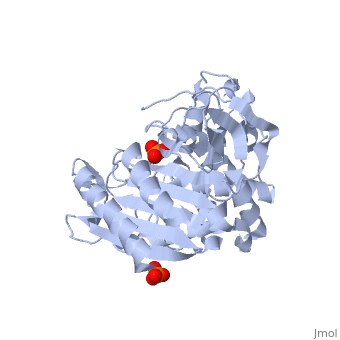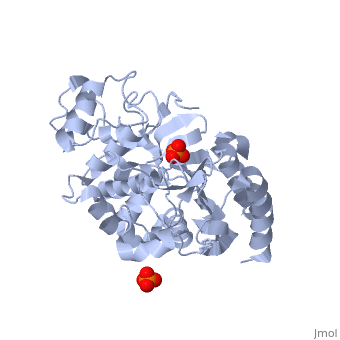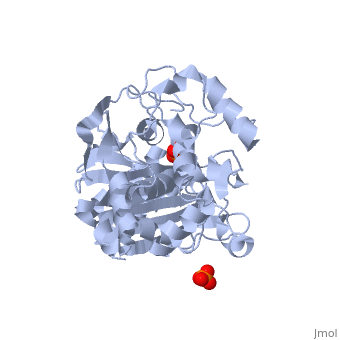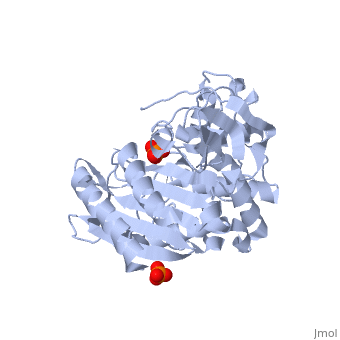
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=> Since the discovery of penicillin in the late 1920s, β-lactam antibiotics, characterized by their central chemical structure, the β-lactam ring, have played an important role in human health (Fig 1). Unfortunately, extensive use, and often misuse, of such drugs has led to an increased resistance in many species of bacterium resulting in major clinical treatment dilemmas. Each year in the United States alone, a minimum of 2 million people are infected with drug-resistant bacteria and of those 2 million, at least 23,000 infections result in fatality.[1]
Clinically, β-lactam antibiotics are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs). PBPs are enzymes that are located in the cell membrane and function in cross-linking to form the peptidoglycan layer. PBPs have a deprotonated serine which executes nucleophilic attack on the carbonyl carbon. The PBP is then covalently attached to one unit of peptidoglycan. The amino group of an alanine on a second unit of peptidoglycan then performs a second nucleophilic attack on the carbonyl carbon, resulting in two covalently cross-linked peptidoglycan units and the regeneration of the catalytic PBP (Fig 2).
[[Image:|500px|right|thumb|Fig 2: Peptidoglycan PBP cross-linking mechanism ]]
The β-lactam ring covalently attaches to PBPs, inhibiting them from executing their role in properly synthesizing the cell wall peptidoglycan layer, via nucleophilic attack of the carbonyl carbon (Fig 3). The β-lactam cannot be removed and thus permanently renders the PBP incapable of its catalytic function in cross-linking. Ultimately, this results in death of bacterial cells from osmotic instability or autolysis
ReferencesReferences
- ↑ Antibiotic Resistant Threat Report in the United States, 2013. Centers for Disease Control and Prevention. 16 September, 2013.