2hh7
| |||||||
| , resolution 2.55Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
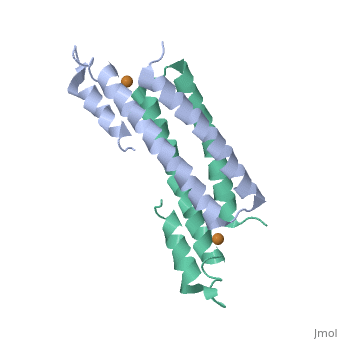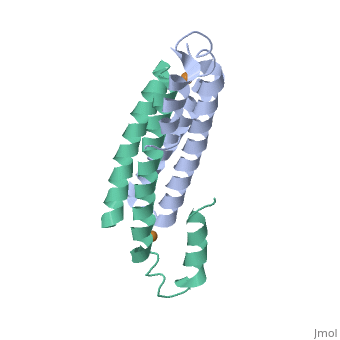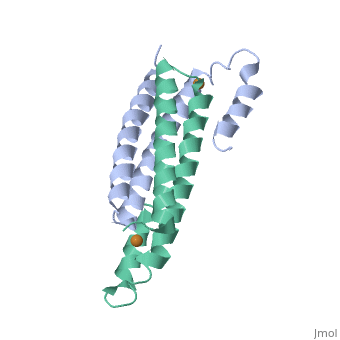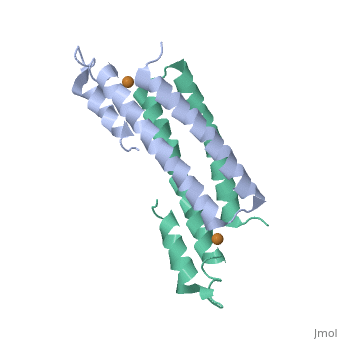
Crystal Structure of Cu(I) bound CsoR from Mycobacterium tuberculosis.
OverviewOverview
Copper is an essential element that becomes highly cytotoxic when concentrations exceed the capacity of cells to sequester the ion. Here, we identify a new copper-specific repressor (CsoR) of a copper-sensitive operon (cso) in Mycobacterium tuberculosis (Mtb) that is representative of a large, previously uncharacterized family of proteins (DUF156). Electronic and X-ray absorption spectroscopies reveal that CsoR binds a single-monomer mole equivalent of Cu(I) to form a trigonally coordinated (S(2)N) Cu(I) complex. The 2.6-A crystal structure of copper-loaded CsoR shows a homodimeric antiparallel four-helix bundle architecture that represents a novel DNA-binding fold. The Cu(I) is coordinated by Cys36, Cys65' and His61' in a subunit bridging site. Cu(I) binding negatively regulates the binding of CsoR to a DNA fragment encompassing the operator-promoter region of the Mtb cso operon; this results in derepression of the operon in Mtb and the heterologous host Mycobacterium smegmatis. Substitution of Cys36 or His61 with alanine abolishes Cu(I)- and CsoR-dependent regulation in vivo and in vitro. Potential roles of CsoR in Mtb pathogenesis are discussed.
About this StructureAbout this Structure
2HH7 is a Single protein structure of sequence from Mycobacterium tuberculosis. Full crystallographic information is available from OCA.
ReferenceReference
CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator., Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP, Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269
Page seeded by OCA on Thu Mar 20 17:17:02 2008