1gzx
OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS
|
OverviewOverview
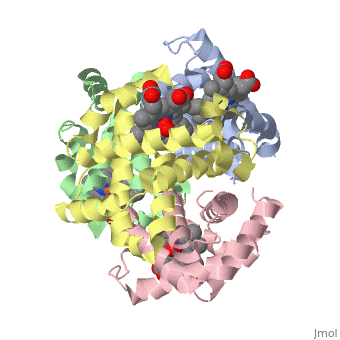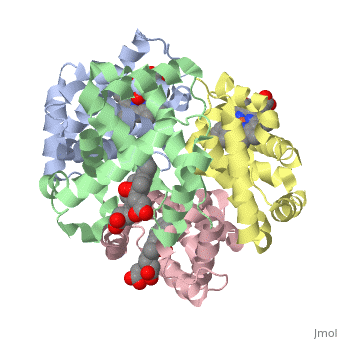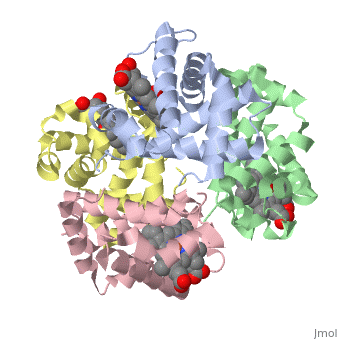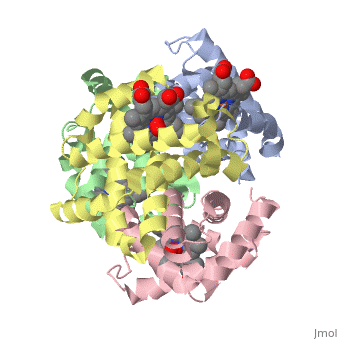
The cooperative binding of oxygen by haemoglobin results from restraints, on ligand binding in the T state. The unfavourable interactions made by, the ligands at the haems destabilise the T state and favour the high, affinity R state. The T <==> R equilibrium leads, in the presence of a, ligand, to a rapid increase in the R state population and therefore, generates cooperative binding. There is now considerable understanding of, this phenomenon, but the interactions that reduce ligand affinity in the T, state have not yet been fully explored, owing to the difficulties in, preparing T state haemoglobin crystals in which all the subunits are, oxygenated. A protocol has been developed to oxygenate deoxy T state adult, human haemoglobin (HbA) crystals in air at 4 C at all four haems without, significant loss of crystalline order. The X-ray crystal structure, determined to 2.1 A spacing, shows significant changes in the alpha and, beta haem pockets as well as changes at the alpha(1)beta(2) interface in, the direction of the R quaternary structure. Most of the shifts and, deviations from deoxy T state HbA are similar to, but larger than, those, previously observed in the T state met and other partially liganded T, state forms. They provide clear evidence of haem-haem interaction in the T, state.
About this StructureAbout this Structure
1GZX is a Protein complex structure of sequences from Homo sapiens with HEM and OXY as ligands. Structure known Active Site: HB1. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structure of T state haemoglobin with oxygen bound at all four haems., Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G, J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:8642597
Page seeded by OCA on Mon Nov 5 12:53:08 2007