1fxx
THE STRUCTURE OF EXONUCLEASE I SUGGESTS HOW PROCESSIVITY IS ACHIEVEDTHE STRUCTURE OF EXONUCLEASE I SUGGESTS HOW PROCESSIVITY IS ACHIEVED
Structural highlights
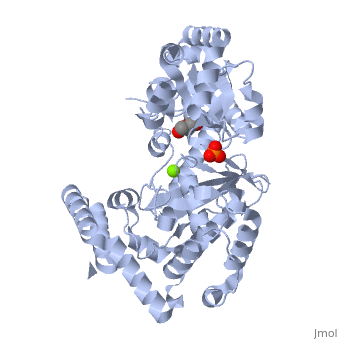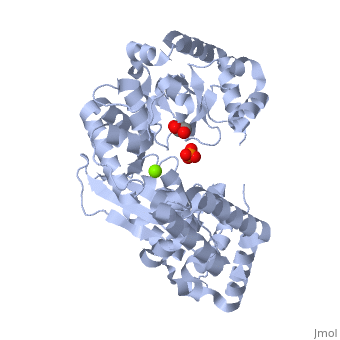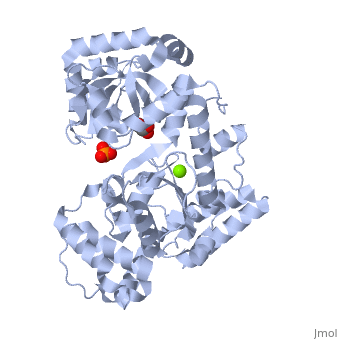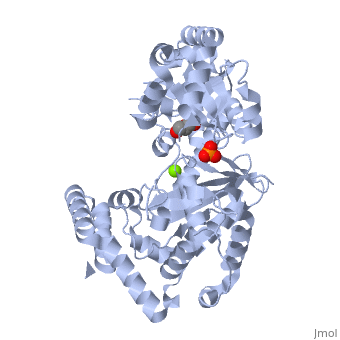
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedExonuclease I (ExoI) from Escherichia coli is a monomeric enzyme that processively degrades single stranded DNA in the 3' to 5' direction and has been implicated in DNA recombination and repair. Determination of the structure of ExoI to 2.4 A resolution using X-ray crystallography verifies the expected correspondence between a region of ExoI and the exonuclease (or proofreading) domains of the DNA polymerases. The overall fold of ExoI also includes two other regions, one of which extends the exonuclease domain and another that can be described as an elaborated SH3 domain. These three regions combine to form a molecule that is shaped like the letter C, although it also contains a segment that effectively converts the C into an O-like shape. The structure of ExoI thus provides additional support for the idea that DNA metabolizing enzymes achieve processivity by completely enclosing the DNA. Structure of Escherichia coli exonuclease I suggests how processivity is achieved.,Breyer WA, Matthews BW Nat Struct Biol. 2000 Dec;7(12):1125-8. PMID:11101894[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||