RuBisCO
Ribulose-1,5-bisphosphate carboxylase oxygenase – RuBisCO (RBCO) catalyzes the first step in photosynthetic carbon fixation, and it is the most abundant protein on earth. RBCO can either carboxylate or oxygenate ribulose-1,5-bisphosphate (RUBP) with CO2 or O2, respectively. RBCO from flowering plants consists of eight large subunits and eight small subunits. Some additional details can be found in Ribulose-1,5-bisphosphate carboxylase/oxygenase.
Quaternery StructureQuaternery Structure
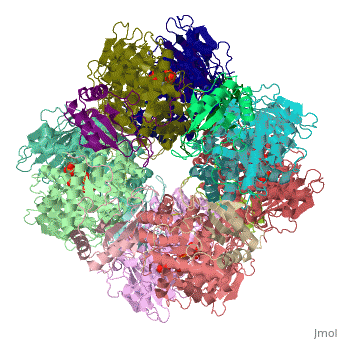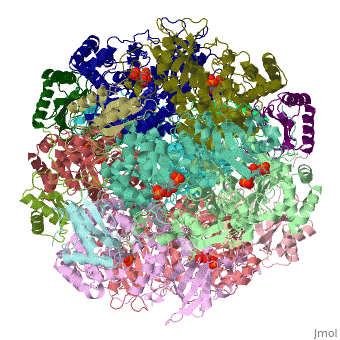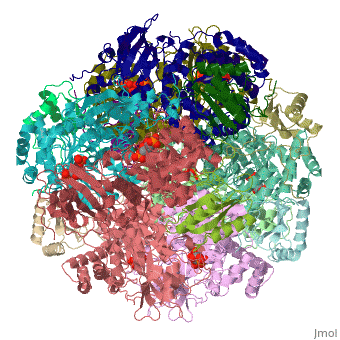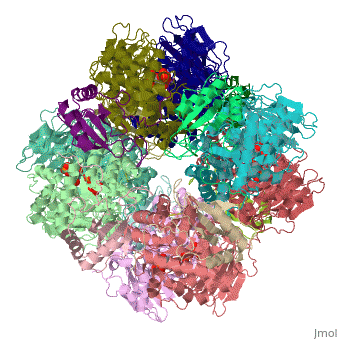
The structure of the Rubisco from spinach is shown in spacefill with its 8 large subunits in shades of blue and and its 8 small subunits in shades of yellow. The large subunits are arranged in head-to-toe pairs like staves in a barrel and the small subunits are arranged at the ends of the barrel. The large subunits contain the active sites and the function of the small subunits is not understood.
Large Subunit StructureLarge Subunit Structure
This isolated shows that each subunit has a large C-terminal lobe and a small N-terminal lobe, and the subunits are arranged head-to-toe (antiparallel). are located in the interface of the large subunit pair. The subunits are shown in cartoon with one shown in the secondary structure color scheme. Each active site is occupied by RUBP, which is shown in CPK spacefill. Here is a showing that both lobes contain alpha helices (pink) and beta strands (yellow). The large lobe is dominated by an (amino acids 166-409), which contributes most of the residues that form the the active site. One residue from the N-terminal lobe of the adjacent large subunit completes the active site. This scene shows RUBP in spacefill and CPK in one of the active sites in the dimer. Both subunits are shown in transparent cartoon with the α-β barrel is pink and yellow. Asn 123 from the adjacent subunit is in blue spacefill, and residues 121-129 are shown in blue cartoon. This residue does not contribute to catalysis, and it will not be considered further.
Active Site StructureActive Site Structure
(under construction)
This scene shows an (cartoon and colored for secondary structure) of spinach Rubisco 8ruc, bound to the naturally occurring inhibitor 2-carboxylarabinitol-1,5-bisphosphate (CAP) and Mg2+, both shown in CPK spacefill.
The structure of CAP (left figure) is similar to the hydrated reaction intermediate that is formed following the addition of CO2 to RUBP. CAP sits at one end of the α-β barrel, and only residues from the beta strands (gold ball & stick) and loops that link them to helices (silver ball & stick) contribute to the (the residue contributed by the N-terminal of the adjacent subunit is not shown).
3D Structures of RuBisCO3D Structures of RuBisCO
Updated February 2013
RuBisCORuBisCO
3rg6, 1rbl – SeRBCO – Synechococcus elongatus
2ybv - RBCO – Thermosynechococcus elongatus
3qfw - RBCO large subunit – Rhodopseudomonas palustris
1uzh, 1gk8 – CrRBCO – Chlamydomonas reinhardtii
1uw9, 1uwa – CrRBCO (mutant)
1svd – RBCD – Halothiobacillus neapolitanus
1bxn – RBCO – Cupriavidus necator
1aus - spRBCO – spinach
1rba - RrRBCO (mutant) – Rhododpirillum rubrum
5rub - RrRBCO
2wvw – RBCO – Anabena – Cryo EM
2vdh, 2vdi, 2v67, 2v68, 2v63, 2v69, 2v6a - CrRBCO (mutant)
1mlv - pRBCO LSMT – pea
2cxe, 2cwx – PhRBCO - Pyrococcus horikoshii
1uzd – CrRBCO/spRBCO
1geh – TkRBCO – Thermococcus kodakaraensis
1iwa - GpRBCO – Galdieria partita
1tel – RBCO large subunit – Chlorobium tepidum
1rld, 3rub, 3t15, 3zw6, 4rub – tRBCO – tobacco
3thg – RBCO – creosote bush
4hhh – RBCO - pea
RuBisCO complex with inhibitor 2-CABPRuBisCO complex with inhibitor 2-CABP
3kdn, 3a12 – TkRBCO III + 2-CABP
3kdo, 3a13 - TkRBCO III (mutant) + 2-CABP
1ir2 - CrRBCO + 2-CABP
1upm, 1upp, 1rbo, 3ruc, 8ruc - spRBCO + 2-CABP + cation
1ir1 - spRBCO + 2-CABP + CO2 + Mg
1wdd – rRBCO + 2-CABP – rice
1bwv - GpRBCO + 2-CABP
1rlc - tRBCO + 2-CABP
RuBisCO complex with productRuBisCO complex with product
1aa1 – spRBCO + phosphoglycerate
1rus - RrRBCO + phosphoglycerate
RuBisCO complex with substrateRuBisCO complex with substrate
1rcx, 1rxo – spRBCO + ribulose-1,5-bisphosphate
9rub - RrRBCO + ribulose-1,5-bisphosphate
1rsc - SeRBCO + xylulose-1,5-bisphosphate
1rco - spRBCO + xylulose-diol-1,5-bisphosphate
3zxw - SeRBCO + carboxyarabinitol-1,5-bisphosphate
RuBisCO complexesRuBisCO complexes
2h21 – pRBCO LSMT + AdoMet
2h23 - pRBCO LSMT + AdoHcy
2h2e, 1ozv, 1p0y - pRBCO LSMT + AdoMet + lysine
2h2j - pRBCO LSMT + sinefungin
2d69 – PhRBCO + sulfate
2rus - RrRBCO + CO2 + Mg
4f0h – GsRBCO + O2 – Galdieria sulphuraria
4f0k - GsRBCO + CO2 + Mg
4f0m - GsRBCO + Mg
1ej7 – tRBCO + phosphate
3axk – rRBCO + NADP
3axm – rRBCO + 6PG