Single stranded binding protein
Sandbox Single Stranded DNA-Binding Protein (SSB)
OverviewOverview
Single-stranded DNA-binding protein, or SSB binds to single-stranded regions of DNA. This binding serves a variety of functions - it prevents the strands from hardening too early during replication, it protects the single-stranded DNA from being broken down by nucleases, and it removes the secondary structure of the strands so that other enzymes are able to access them and act effectively upon the strands[1].
Single-stranded DNA (ssDNA) is utilized primarily during the course of major aspects of DNA metabolism such as replication, recombination and repair (PMID: 2087220). In addition to stabilizing ssDNA, SSB proteins also bind to and control the function of many other proteins that are involved in all of three of these major DNA metabolic processes. During DNA replication, SSB molecules bind to the newly separated individual DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template for new DNA synthesis[2].
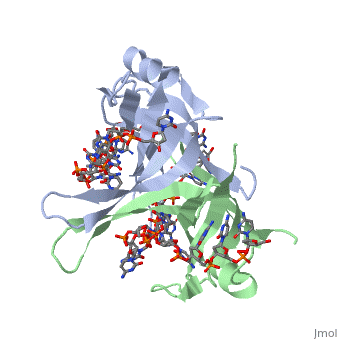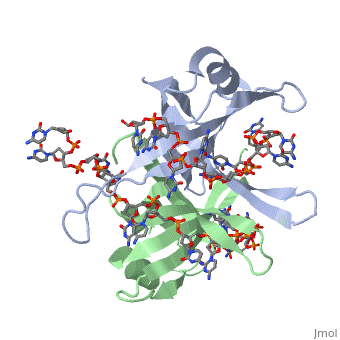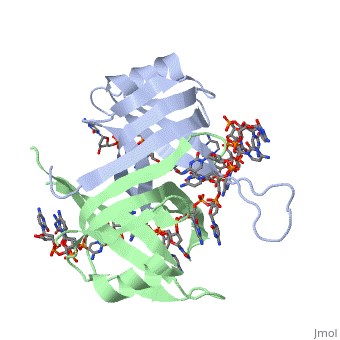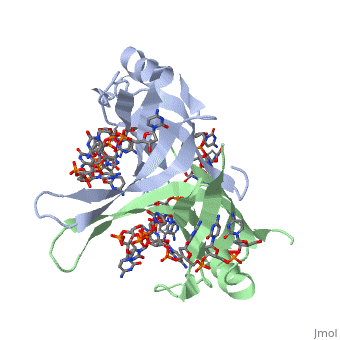
Structure of E. coli SSBStructure of E. coli SSB
SSB proteins have been identified in many different organisms, but the most well understood SSB remains the SSB of E. coli. E. coli SSB is a homotetramer consisting of four identical subunits which are each about 19 kDa in size [3]. There are two different binding modes of the E. coli SSB when it complexes with ssDNA (Lohman). Regulation of these modes has been found to be dependent on salt concentration, in addition to other unknown factors. Under low salt conditions, the protein is less efficient as only two of the four identical subunits of E. coli SSB were found to bind to the ssDNA [4]. Under high salt concentrations, however, all four subunits of the homotetramer bind to the ssDNA, increasing the number of nucleotides in contact with the SSB and thus favoring SSB-ssDNA interactions. Depending on the salt concentration and other factors, estimates of the size of the site of interaction between SSB and ssDNA range anywhere from 30 to 73 nucleotides for each tetramer [5]. Active E. coli SSB is made of a homotetramer with extensive DNA binding domains that bind to a single strand of DNA (PMID: 2087220). The tetramers consist of α-helices, β-sheets, and random coils. Each subunit contains an α-helix and several β-sheets. The secondary structure also includes a NH2 terminus, which consists of multiple positively charged amino acids. The DNA-binding domain lies within 115 amino acid residues from this terminus. The COOH terminus includes many acidic amino acids (PMID: 2087220). |
| ||||||||||
StructureStructure
|
SSB consists is a homotetramer that has a DNA binding domain which binds to a single strand of DNA. The tetramers consist of α-helices, β-sheets, and random coils. Each subunit contains an and several . The secondary structure also includes a NH2 terminus, which consists of multiple positively charged amino acids. The DNA-binding domain lies within 115 amino acid residues from this terminus [6]. The COOH terminus includes many acidic amino acids. Binding Interactions in the Active SitessDNA can interact with binding proteins through hydrogen bonds, stacking, or electronegative interactions. Most interactions between SSB and ssDNA happen through the OB fold. OB stands for oligosaccharide/oligonucleotide binding site. This fold consists of a 5-stranded β barrel that ends in an α-helix. is an important DNA binding site. It has been shown to be the site for cross-linking. Tryptophan and Lysine residues are important in binding as well. Treatments resulting in modification of arginine, cysteine, or tyrosine residues had no effect on binding of SSB to DNA, whereas modification of either lysine residues (with acetic anhydride) or tryptophan residues (with N-bromosuccinimide) led to complete loss of binding activity [7]. SSB-Protein InteractionsSSB can form complexes with many other proteins. This trait can keep enzymes needed for damage repair, transcription, etc. near the ssDNA and it is thought that SSB can even help to stimulate these enzymes to carry out their jobs. When DNA binds SSB, most of the molecule loses flexibility. But the COOH terminal domain remain flexible, even after DNA binding. It is believed that the COOH terminus has something to do with protein binding [8]. SSB will interact with the protein RecA to enable recombination, because RecA will recognize SSB and replace it on the strand. In DNA repair, SSB will bind to the damaged strand to protect it. And eventually it will attract repair enzymes which will replace SSB and begin repair mechanisms. SSB has also been thought to bind with exonuclease I, DNA polymerase II, and a protein n, which is used to help synthesize RNA primers for the lagging strand. SSB can also help regulate transcription by competing with other proteins for binding spaces on DNA. SSB has a higher affinity for DNA than most other proteins, and those proteins are not able to remove SSB from DNA and bind themselves. This type of mechanism can not only regulate transcription, but it can provide protection for the DNA [9]. | ||||||||||
Binding Interactions between DNA and SSB of E. coliBinding Interactions between DNA and SSB of E. coli
Phe60 is an important DNA binding site. It has been shown to be the site for cross-linking. and residues are important in binding as well. Treatments resulting in modification of arginine, cysteine, or tyrosine residues had no effect on binding of SSB to DNA, whereas modification of either lysine residues (with acetic anhydride) or tryptophan residues (with N-bromosuccinimide) led to complete loss of binding activity [10]. The two tryptophan residues involved in DNA binding are and Trp54, which was determined by mutagenesis. One more binding site was determined by site-specific mutagenesis. When His55 is substituted with Leu it decreases binding affinity. All of these residues are found in a hydrophobic region, which is suitable for nucleotide base interactions. SSB-Protein InteractionsMost of the molecule loses flexibility after DNA binding. But three of the phenylalanines (147, 171, 177) in the COOH terminal domain remain flexible, even after DNA binding. It is believed that the COOH terminus has something to do with protein binding [11]. One experiment in which Phe-177 was changed to Cys resulted in a protein that could not replicate DNA. This replication defect results from the inability of C-terminus to bind to other replication proteins [12]. It is believed that may play an important role in binding the RecA protein. Mutations in Gly15 have extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, and a protein n, which is used to help synthesize RNA primers for the lagging strand.
|
| ||||||||||
See AlsoSee Also
ReferencesReferences
- ↑ PMID: 2087220)
- ↑ Berg JM, Tymoczko JL, Stryer L. Biochemistry. 6th edition. New York: W H Freeman; 2006.
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Kozlov AG, Lohman TM. Stopped-flow studies of the kinetics of single-stranded DNA binding and wrapping around the Escherichia coli SSB tetramer. Biochemistry. 2002 May 14;41(19):6032-44. PMID:11993998
- ↑ Kozlov AG, Lohman TM. Stopped-flow studies of the kinetics of single-stranded DNA binding and wrapping around the Escherichia coli SSB tetramer. Biochemistry. 2002 May 14;41(19):6032-44. PMID:11993998
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Agamova KA, Gladunova ZD, Savinkin IuN. [Cytologic method in the diagnosis of precancerous conditions and early cancer of the stomach]. Lab Delo. 1988;(3):43-5. PMID:2453719