1uyn
TRANSLOCATOR DOMAIN OF AUTOTRANSPORTER NALP FROM NEISSERIA MENINGITIDIS
|
OverviewOverview
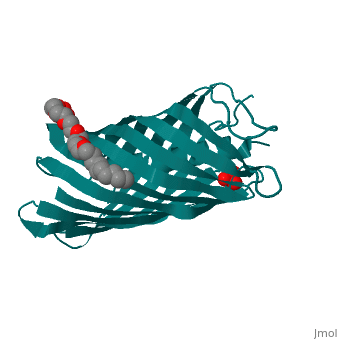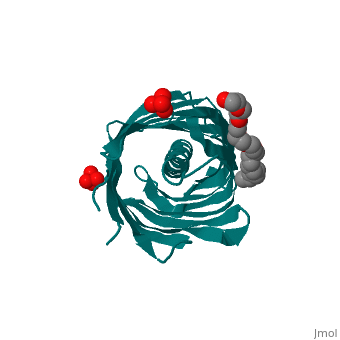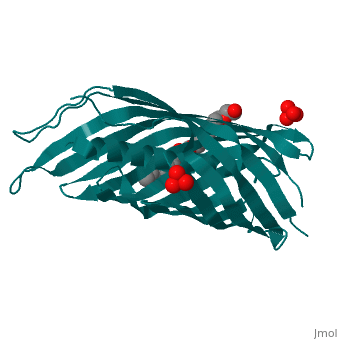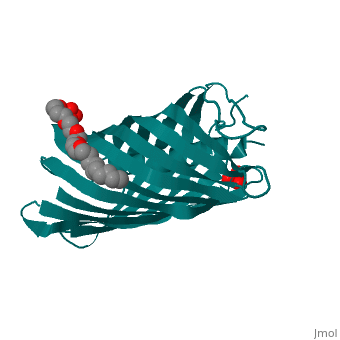
Autotransporters are virulence-related proteins of Gram-negative bacteria, that are secreted via an outer-membrane-based C-terminal extension, the, translocator domain. This domain supposedly is sufficient for the, transport of the N-terminal passenger domain across the outer membrane. We, present here the crystal structure of the in vitro-folded translocator, domain of the autotransporter NalP from Neisseria meningitidis, which, reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A, that is filled by an N-terminal alpha-helix. The domain has pore activity, in vivo and in vitro. Our data are consistent with the model of, passenger-domain transport through the hydrophilic channel within the, beta-barrel, and inconsistent with a model for transport through a central, ... [(full description)]
About this StructureAbout this Structure
1UYN is a [Single protein] structure of sequence from [Neisseria meningitidis] with SO4 and CXE as [ligands]. Structure known Active Site: AC1. Full crystallographic information is available from [OCA].
ReferenceReference
Structure of the translocator domain of a bacterial autotransporter., Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P, EMBO J. 2004 Mar 24;23(6):1257-66. Epub 2004 Mar 11. PMID:15014442
Page seeded by OCA on Tue Oct 30 16:15:37 2007