G18secL03Tpc4
|
Outer surface protein C (OspC) of Borrelia burgdorferiOuter surface protein C (OspC) of Borrelia burgdorferi
Outer surface protein C (OspC) is one of the major antigens on the surface of the Lyme disease spirochete, Borrelia burgdorferi, along with other outer surface proteins A and B (OspA and OspB, respectively). It greatly differs from OspA and OspB in both structure and function. The uniqueness of OspC is that it comes into play when the pathogen is being transmitted to humans or other mammals.OspC is critical for survival in or transmission to the tick or mammalian host.[1] OspC is being produced by Borrelia burgdorferi during a very short time interval when infected ticks start feeding, but its synthesis is known to slow down greatly after transmission to a mammalian host. It was demonstrated that those spirochetes that lack OspC are capable to replicate inside and migrate to the salivary glands of the tick vector but do not infect mammals. [2] Without OspC the spirochetes are believed to be unable to adapt to the environment inside the host. Therefore, OspC is believed to determine virulence of the spirochete to mammals, including humans.
Basic Structure DescriptionBasic Structure Description
- Primary Structure
The ospC gene is located on a 27 kb circular plasmid and encodes a lipoprotein of 22–23 kDa.[3] The protein is initially synthesized with an 18-amino-acid-long signal sequence which is removed during processing and lipidation at the amino proximal Cys residue. OspC proteins are highly polymorphic and this variability extends even to strains collected from a single geographical area.[4]
- Secondary Structure
OspC is predominantly α-helical in its secondary structure. β-sheets are also present, but they are rather short and not promininent.
- Tertiary Structure
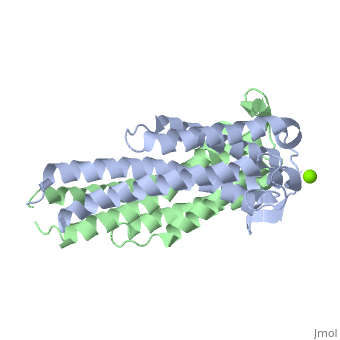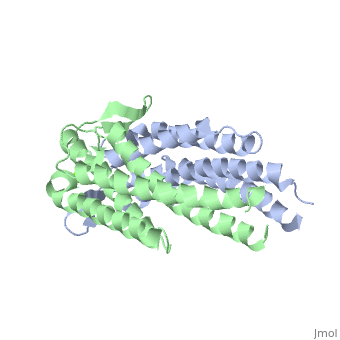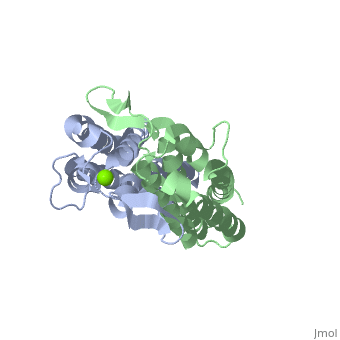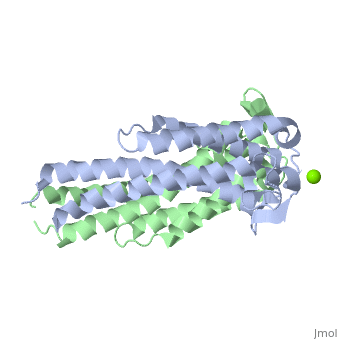
A single OspC monomer subunit is composed of 4 long and 1 short α-helices. Also, 2 short segments of β-sheets are observed near the binding site of the molecule.
- Quaternary Structure
OspC is a dimerized molecule, with 2 identical monomeric subunits comprising a dimer. However, for a binding event to occur, 2 dimers are necessary.
Major Hypothesized FunctionsMajor Hypothesized Functions
- Adaptation and survival in different host environments
Borrelia burgdorferi expresses OspA but not OspC when residing in the midgut of unfed ticks. However, when the tick starts feeding on mammals, OspC synthesis is induced and OspA is repressed.The switch is in part due to the change in temperature; OspC is induced at 32–37°C, but not at 24°C, and this upregulation is at the transcriptional and translational levels. Evidence suggests co-regulation of these two genes at the mRNA level. Clearly, to survive in both hosts, spirochetes have evolved mechanisms for sensing the different host environments and responding accordingly.[5]
- Binding
Although four helical bundle structures may have a variety of functions which are to be defined in further research, OspC may possibly be a binding protein contributing to a fundamental biological process. Several studies have shown that B.burgdorferi has a predilection for collagenous tissue and can interact with fibronectin and cellular collagens. The spirochetes can bind to a number of different cell types, including fibroblasts. Borrelia burgdorferi can bind to a novel circulating fibroblast-like cell called the peripheral blood fibrocyte, which expresses collagen types I and III as well as fibronectin, in a process that does not require OspA or OspB.[6]
Putative Binding SitePutative Binding Site
Role of OspC in Lyme DiseaseRole of OspC in Lyme Disease
OspC-based Vaccine Against Lyme DiseaseOspC-based Vaccine Against Lyme Disease
Main Advantages of Developing OspC-based VaccineMain Advantages of Developing OspC-based Vaccine
Main Problems with Application of OspC-based VaccineMain Problems with Application of OspC-based Vaccine
Notes and References
- ↑ Stewart PE, Thalken R, Bono JL, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001 Feb;39(3):714-21. PMID:11169111
- ↑ D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [1]
- ↑ Marconi RT, Samuels DS, Garon CF. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926-32. PMID:7679385
- ↑ D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [2]
- ↑ D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [3]
- ↑ Grab DJ, Lanners H, Martin LN, Chesney J, Cai C, Adkisson HD, Bucala R. Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol Med. 1999 Jan;5(1):46-54. PMID:10072447