1b3q
|
CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE
OverviewOverview
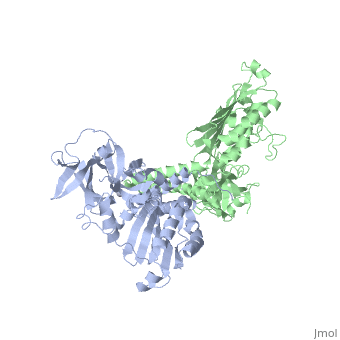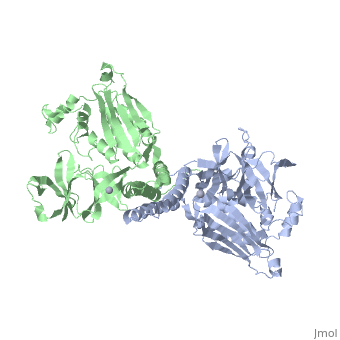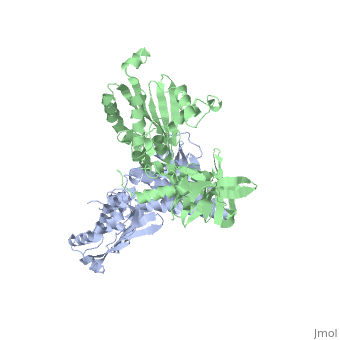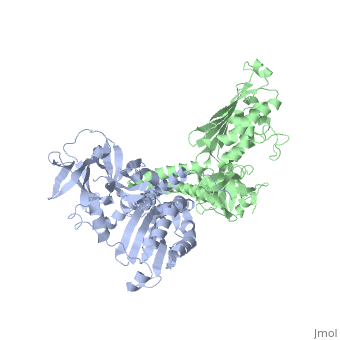
Histidine kinases allow bacteria, plants, and fungi to sense and respond to their environment. The 2.6 A resolution crystal structure of Thermotoga maritima CheA (290-671) histidine kinase reveals a dimer where the functions of dimerization, ATP binding, and regulation are segregated into domains. The kinase domain is unlike Ser/Thr/Tyr kinases but resembles two ATPases, Gyrase B and Hsp90. Structural analogies within this superfamily suggest that the P1 domain of CheA provides the nucleophilic histidine and activating glutamate for phosphotransfer. The regulatory domain, which binds the homologous receptor-coupling protein CheW, topologically resembles two SH3 domains and provides different protein recognition surfaces at each end. The dimerization domain forms a central four-helix bundle about which the kinase and regulatory domains pivot on conserved hinges to modulate transphosphorylation. Different subunit conformations suggest that relative domain motions link receptor response to kinase activity.
About this StructureAbout this Structure
1B3Q is a Single protein structure of sequence from Thermotoga maritima with as ligand. Full crystallographic information is available from OCA.
ReferenceReference
Structure of CheA, a signal-transducing histidine kinase., Bilwes AM, Alex LA, Crane BR, Simon MI, Cell. 1999 Jan 8;96(1):131-41. PMID:9989504
Page seeded by OCA on Thu Feb 21 11:51:06 2008