CAP-Gly domain
| |||||||||
| 1tov, resolution 1.77Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | F53F4.3 (Caenorhabditis elegans) | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
CAP-Gly domains in cytoskeletal proteinsCAP-Gly domains in cytoskeletal proteins

ActivityActivity
The cytoskeletal network contains Cytoskeleton-Associated Proteins (CAPs), including CLIP-170 and dynactins, that function in the organization of microtubules and in the transportation of vesicles and organelles. CAPs that bind to the +end of microtubules generally contain one or more glycine-rich domains ("CAP-Gly" domains) with a well-conserved GKNDG sequence motif[1], presumably involved in protein-protein binding.
StructureStructure
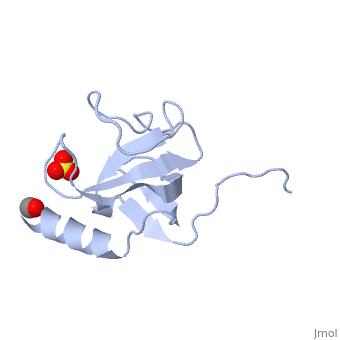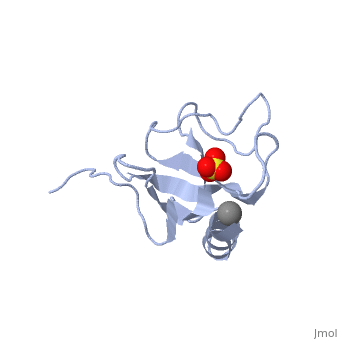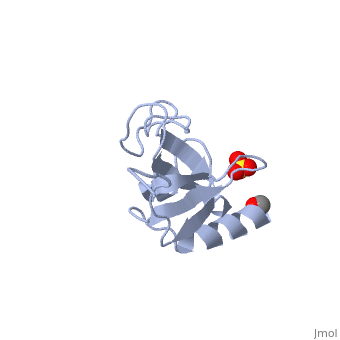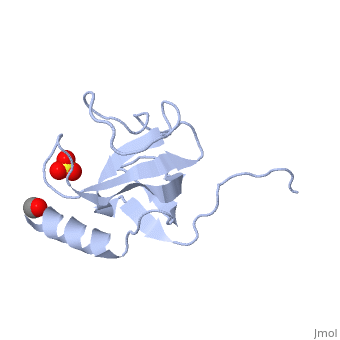
The first crystal structure of a CAP-Gly domain (PDP file 1lpl), from C. elegans protein F53F4.3, revealed a novel protein fold containing a small 5-strand antiparallel beta-barrel, several loops, and an N-terminal helix. The GKNDG sequence is in two consecutive turns of a surface loop, at one side of a groove (as shown in image at left). The groove is lined by a large, concave patch of residues highly conserved among the related sequences[2]. In the crystal, a dimer is formed by binding of the C-terminus of each chain into the GKNDG groove of the other chain.
1TOV is a rebuilt and re-refined version of the same dataset from 1LPL, giving an improvement of about 4% in Rfree, identifying a sulfate near the groove (seen in both 2D and interactive images), and adding 3 more residues of ordered helix toward the N-terminal end of the domain fragment. Both are from the SouthEast Collaboratory for Structural Genomics (SECSG), solved by single wavelength sulfur-anomalous phasing. The new 1tov structure was produced as part of a systematic 30-structure test of the then-new MolProbity methods for diagnosing and correcting problems in crystallographic models[3].
ReferencesReferences
- ↑ Riehemann K, Sorg C. Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem Sci. 1993 Mar;18(3):82-3. PMID:8480366
- ↑ Li S, Finley J, Liu ZJ, Qiu SH, Chen H, Luan CH, Carson M, Tsao J, Johnson D, Lin G, Zhao J, Thomas W, Nagy LA, Sha B, DeLucas LJ, Wang BC, Luo M. Crystal structure of the cytoskeleton-associated protein glycine-rich (CAP-Gly) domain. J Biol Chem. 2002 Dec 13;277(50):48596-601. Epub 2002 Sep 7. PMID:12221106 doi:http://dx.doi.org/10.1074/jbc.M208512200
- ↑ Arendall WB 3rd, Tempel W, Richardson JS, Zhou W, Wang S, Davis IW, Liu ZJ, Rose JP, Carson WM, Luo M, Richardson DC, Wang BC. A test of enhancing model accuracy in high-throughput crystallography. J Struct Funct Genomics. 2005;6(1):1-11. PMID:15965733 doi:10.1007/s10969-005-3138-4