To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
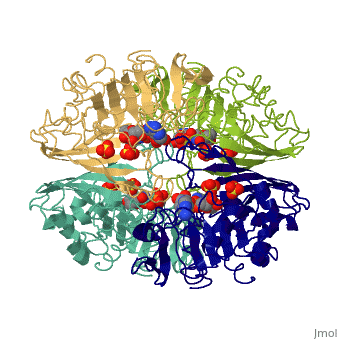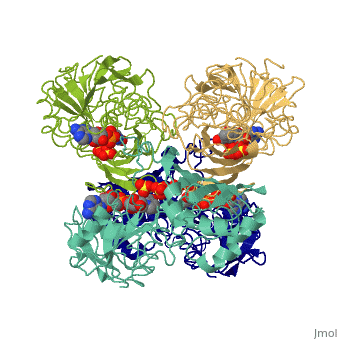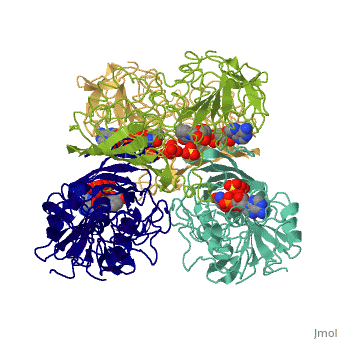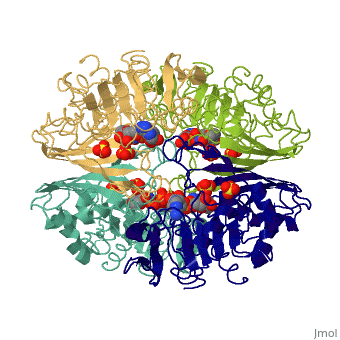
Glyceraldehyde-3-phosphate Dehydrogenase
(abbreviated as GAPDH or the less common G3PDH) (EC 1.2.1.12) ~37kDa is a well-known enzyme which catalyzes the sixth step of glycolysis, a reversible cytosolic process in eukaryotes which involves the breakdown of glucose for energy and carbon molecules. Along with its role in glycolysis and gluconeogenesis, recent research has determined that GAPDH is actually a multifunctional protein, as it has numerous defined, non-metabolic functions involved in multiple subcellular processes including transcription activation, ER to Golgi transportation, transcriptional control of histone gene expression, nuclear membrane fusion, neuronal initiation of apoptosis, recognizing fraudulently incorporated nucleotides in DNA, and maintaining telomere structures. Research also shows that it possibly has a direct involvement in cellular phenotype of human neurodegenerative disorders, especially those characterized by expansion of CAG repeats.
Role in Glycolysis:
- In two coupled steps,
 GAPDH catalyzes the conversion of glyceraldyhyde-3-phosphate at carbon 1 to 1,3-bisphosphoglycerate (1,3-BPG).
GAPDH catalyzes the conversion of glyceraldyhyde-3-phosphate at carbon 1 to 1,3-bisphosphoglycerate (1,3-BPG).
- The reaction involving the GAPDH enzyme combines phosphorylation with oxidation in an overall endergonic reaction. (ΔG°'=+6.3 kJ/mol (+1.5 kcal/mol)) First, the oxidation of glyceraldyhyde-3-phosphate to D-glycerate 1,3-bisphosphate takes place, in which an aldehyde is converted to carboxylic acid ((ΔG°'=-50 kJ/mol (-12 kcal/mol))and NAD+ (Nicotinamide adenine dinucleotide), an important co-factor and ligand found bound to the of GAPDH, is simultaneously reduced endergonically to NADH. This oxidation reaction is required for the initiation of the second reaction because it is highly exergonic and thus drives the endergonic second reaction ((ΔG°'=+50 kJ/mol (+12 kcal/mol)). In the second reaction a molecule of inorganic phosphate is transferred to a GAP intermediate to form a product with a high potential to transfer phosphates, 1,3-bisphosphoglycerate.
- The mechanism of GAPDH is mainly dependent the thiol functional group on a cysteine residue which lies within the . This important thiol group acts as a nucleophile, attacking the carbon on the aldehyde functional group of the substrate, glyceraldehyde 3-phosphate, after it binds to GAPDH. The creation of a thiohemiacetal intermediate occurs from this oxidative reaction, which then loses a hydride to NAD+, which is also bound nearby, forming NADH and a carboxyl group from the aldehyde. Conservation of energy is maintained through this thioester linkage to the active site cysteine. The GAP intermediate is then used in the second step, where it is important to notice that without GAPDH's use of covalent catalysis, the energy barrier of the reaction would be too high and the reaction would be too slow for living organisms.
Other roles:
- The importance of GAPDH in transcription was discovered by Zheng et. al. in 2003. It was found that OCA's transcriptional coactivator complex contains GAPDH, which moves between the cytosol and nucleus and may link the metabolic state to gene transcription.
- In 2005 the initiation of apoptosis was shown to be mediated by GAPDH by Hara et. al. when it was found to bind to DNA like it does in transcription activation.
GAPDH was also found to be involved in ER to Golgi transport because it is recruited by rab2 to vesicular-tubular clusters of the endoplasmic reticulum where it helps form COP 1 vesicles.
Use in the lab:
- Overall, GAPDH is a “housekeeping gene” and is found in high levels in tissues and cells so it is commonly used in biological research as a loading control in western blot and RT-PCR, but it has to be carefully controlled because under specific conditions it can have various regulation.


ReferencesReferences
- ↑"GAPDH glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] - Gene - NCBI." National Center for Biotechnology Information. N.p., n.d. Web. 1 May 2012. Cite error: Closing
</ref> missing for <ref> tag
- ↑"Glyceraldehyde 3-phosphate dehydrogenase - Wikipedia, the free encyclopedia." Wikipedia, the free encyclopedia. N.p., n.d. Web. 1 May 2012. [1]
- ↑"Glyceraldehyde-3-phosphate Dehydrogenase." Biochemistry Dictionary. N.p., n.d. Web. 1 May 2012. [2]
- ↑Isupov, M.N., and J.A. Littlechild. "Glyceraldehyde-3-phosphate Dehydrogenase." RCSB Protein Data Bank. N.p., 8 Oct. 1999. Web. 29 Apr. 2012. [3]
- ↑Minter, Melissa. "Glyceraldehyde-3-phosphate Dehydrogenase." Oxidoreductases and the Reactions they Catalyze. N.p., n.d. Web. 20 Apr. 2012. [4]
- ↑"Structure, Function, and Thermostability of GAPDH." GAPDH. N.p., n.d. Web. 28 Apr. 2012. [5]
- ↑Watson, H.C., and J.C. Campbell. "Twinning In Crystals Of Human Skeletal Muscle D-Glyceraldehyde-3-Phosphate Dehydrogenase." RCSB Protein Data Bank. N.p., 27 Oct. 1983. Web. 28 Apr. 2012. [6]
|