Fadel A. Samatey Group
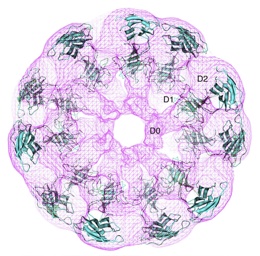 | |
| Crystal structure of flagellar hook fitted into electron density map obtained by electron cryomicroscopy[1]. |
Fadel A. Samatey is Head of the Transmembrane Trafficking Unit at the Okinawa Institute of Science and Technology (OIST) (Japan). Samatey's group uses X-ray crystallography, genetic and biochemical approaches to elucidate the structures and functions of transmembrane proteins, especially type III secretion proteins in bacterial flagella.
Below are listed contributions from the Samatey Group, most recent first.
Interests and ObjectivesInterests and Objectives
Motility is a very important function in the living world. For this purpose, organisms such as bacteria have developed the most incredible molecular machine: the flagellar system. Bacteria such as Escherichia coli and Salmonella typhimurium swim by rotating long helical filaments called the flagellum. The flagellum is a complex structure made by the association of many different proteins. It can be divided into three parts: 1) the filament: a long, rigid, tubular structure that works as a helical propeller, 2) the hook: a short, highly flexible tubular segment that works as a universal joint, and 3) the basal body: a rotary motor embedded in the cell membrane.
During the assembly of the flagellum, all the flagellar axial proteins are exported from the cytoplasm to the flagellum distal end through a 2 -3 nm channel located at its centre. This export mechanism is regulated by a specialized protein export system located on the cytoplasmic side of the basal body. It is called the Type III Export Apparatus and is found throughout the bacterial kingdom. In the case of Salmonella, this export apparatus is made by six membrane proteins: FlhA, FlhB, FliO, FliP, FliQ, FliR, and three cytoplasmic proteins: FliI, FliH and FliJ. The export apparatus of the bacterial flagellum is homolog to the type III secretion system (T3SS) found in Gram-negative pathogenic bacteria. The T3SS role is to secrete virulence factors to host cells, leading to diverse diseases. To understand both the bacterial flagellum and its export apparatus, we have been doing structural studies on some flagellar proteins and genetic studies on the export apparatus.
Contact: f.a.samatey at oist jp
Contributions from OISTContributions from OIST
Samatey joined OIST in 2007.
- ↑ Kido Y, Yoon YH, Samatey FA. Crystallization of a 79 kDa fragment of the hook protein FlgE from Campylobacter jejuni. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Dec 1;67(Pt, 12):1653-7. Epub 2011 Nov 30. PMID:22139190 doi:10.1107/S1744309111043272
- ↑ Meshcheryakov VA, Krieger I, Kostyukova AS, Samatey FA. Structure of a tropomyosin N-terminal fragment at 0.98 A resolution. Acta Crystallogr D Biol Crystallogr. 2011 Sep;67(Pt 9):822-5. Epub 2011, Aug 9. PMID:21904035 doi:10.1107/S090744491102645X
- ↑ Meshcheryakov VA, Samatey FA. Purification, crystallization and preliminary X-ray crystallographic analysis of the C-terminal cytoplasmic domain of FlhB from Salmonella typhimurium. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Jul 1;67(Pt, 7):808-11. Epub 2011 Jun 30. PMID:21795800 doi:10.1107/S1744309111018938
- ↑ Meshcheryakov VA, Yoon YH, Samatey FA. Purification, crystallization and preliminary X-ray crystallographic analysis of the C-terminal cytoplasmic domain of FlhB from Aquifex aeolicus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Feb 1;67(Pt, 2):280-2. Epub 2011 Jan 27. PMID:21301106 doi:10.1107/S1744309110052942
- ↑ Barker CS, Meshcheryakova IV, Kostyukova AS, Samatey FA. FliO regulation of FliP in the formation of the Salmonella enterica flagellum. PLoS Genet. 2010 Sep 30;6(9). pii: e1001143. PMID:20941389 doi:10.1371/journal.pgen.1001143
Samatey Contributions Prior to OISTSamatey Contributions Prior to OIST
Before joining OIST, from 1996-2007 Samatey was a member of the Keiichi Namba Group, from 1996 at Matsushita Electric, from 1997 in the ERATO Protonic Nanomachine Project, and from 2002 at Osaka University, Japan. Samatey earned his Ph.D. in 1992 at Université Joseph Fourier in Grenoble, France.
 | |
| Simplified model of the rotating bacterial flagellar hook. |
- ↑ Furuta T, Samatey FA, Matsunami H, Imada K, Namba K, Kitao A. Gap compression/extension mechanism of bacterial flagellar hook as the molecular universal joint. J Struct Biol. 2007 Mar;157(3):481-90. Epub 2006 Oct 20. PMID:17142059 doi:10.1016/j.jsb.2006.10.006
Analyses the mechanism by which monomers of the flagellar hook slide against each other during hook rotation, to permit bending while transmitting torque.
- ↑ Kitao A, Yonekura K, Maki-Yonekura S, Samatey FA, Imada K, Namba K, Go N. Switch interactions control energy frustration and multiple flagellar filament structures. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):4894-9. Epub 2006 Mar 20. PMID:16549789 doi:10.1073/pnas.0510285103
Describes a massive molecular dynamics simulation that successfully accounts for polymorphic supercoiling in the bacterial flagellar filament. Interactions between protein monomer chains are dissected into permanent, sliding, and switch categories.
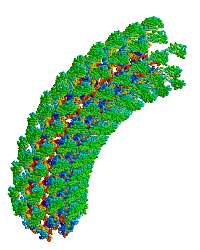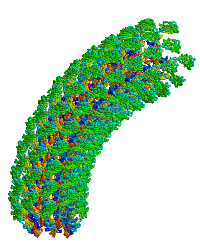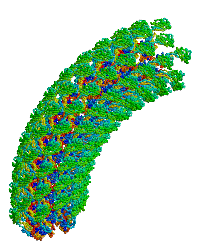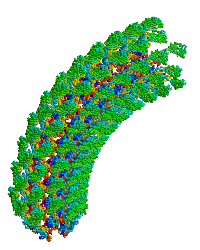 | |
| Bacterial flagellar hook. |
|
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Shaikh TR, Thomas DR, Chen JZ, Derosier DJ, Kitao A, Namba K. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature. 2004 Oct 28;431(7012):1062-8. PMID:15510139 doi:http://dx.doi.org/10.1038/nature02997
Reports the first structure of a major fragment of the protein monomer that assembles into the bacterial flagellar hook (1wlg, 1.8 Å resolution). Fits the monomer into an electron cryomicroscopic density map, resulting in straight and curved models of the hook, including a rotating model.
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Namba K. Crystallization of a core fragment of the flagellar hook protein FlgE. Acta Crystallogr D Biol Crystallogr. 2004 Nov;60(Pt 11):2078-80. Epub 2004, Oct 20. PMID:15502333 doi:10.1107/S0907444904022735
|
- ↑ Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001 Mar 15;410(6826):331-7. PMID:11268201 doi:10.1038/35066504
Reports, for the first time, the atomic structure of a major fragment of the protein chain monomer that assembles into the bacterial flagellar filament (1io1, 2.0 Å resolution). The crystal contained chains of monomers, which revealed how the monomer protein chains fit together into protofilaments. Theoretical simulation revealed a possible mechanism for how the filament reverses direction, a mechanism crucial to how bacteria swim towards food or away from harm by reversing the flagellar motor.
- ↑ Samatey FA, Imada K, Vonderviszt F, Shirakihara Y, Namba K. Crystallization of the F41 fragment of flagellin and data collection from extremely thin crystals. J Struct Biol. 2000 Nov;132(2):106-11. PMID:11162732 doi:10.1006/jsbi.2000.4312
 | Crystals of the flagellar hook protein FlgE from C. jejuni produced in the Samatey lab. |
1990's1990's
- ↑ Lazzaroni JC, Vianney A, Popot JL, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995 Feb 10;246(1):1-7. PMID:7853390 doi:http://dx.doi.org/10.1006/jmbi.1994.0058
See AlsoSee Also
ReferencesReferences
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Shaikh TR, Thomas DR, Chen JZ, Derosier DJ, Kitao A, Namba K. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature. 2004 Oct 28;431(7012):1062-8. PMID:15510139 doi:http://dx.doi.org/10.1038/nature02997