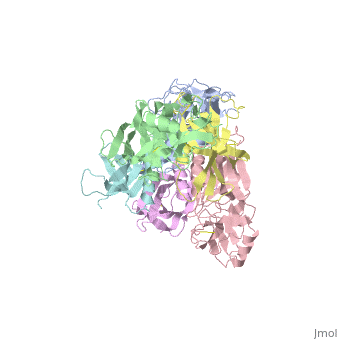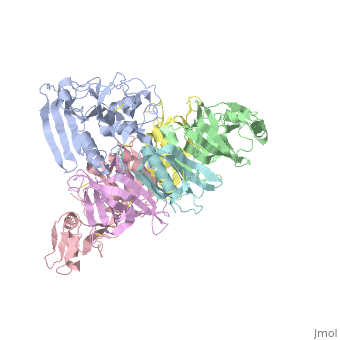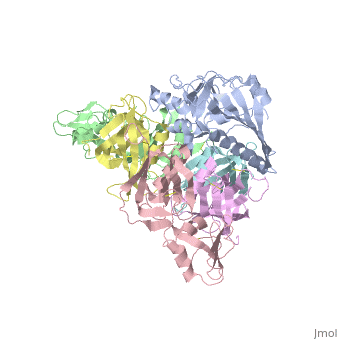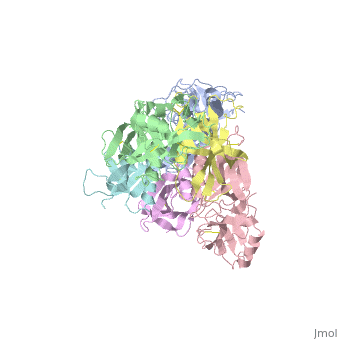Pertussis Toxin-ATP Complex
|
|
introductionintroduction
Protussis Toxins is a major virulence factor of Bordetella pertussis that cause whooping cough. Whooping cough, also known as pertusis, is a highly contagious bacterial disease caused by the bacteria Bordetella pertussis. This disease had been characterized by severe cough that has been documented to cause subconjunctival hemorrhages, rib fractures, hernias, fainting and vertebral artery dissection. The pertussis toxin has been characterized as being a AB toxin meaning that there are 2 subunits: A subunit possesses the enzyme activity and the B subunit it the receptor binding portion. Together this AB toxin colonizes the respiratory tract and becomes activated by destabilization due to the binding of ATP.
structurestructure
The protussis toxin is a AB5 toxin consisting of a six-component protein complex. With that in mind, this protein is a hexamer containing a catalytic (S1) subunit that is tightly associated with the pentameric cell-binding component (B-oligomer). The S1 component is a single subunit while the B-oligomer is a pentamer composed of four types of subunits: , , two copies of , and . This B subnit is what binds to the terminal sialic acid residues.
Pertussis Toxin activationPertussis Toxin activation
Pertussis Toxin by itself is harmless unless activated. From studies, it has became clear that there is a direct interaction between ATP and pertussis toxin which leads to activation. The direct effect of ATP is to destabilize the interaction between the S1 subunit and the B-oligomer by binding to the B-oligomer. This then relaxes the toxin by facilitating the subsequent reduction of a disulphide bond in the S1 subunit. The main interaction that leads to the destabilization is the favorable hydrogen bonding and electrostatic interaction between the triphosphate moiety and five positively charged amino acids: . In contrast, the negatively charged carboxyl terminus of subunit S1 interacts unfavorably with the negative charges of the triphosphate moiety, causing a displacement of the C-terminal of therefore, the repulsion between the triphosphate moiety and the C terminus of subunit S1 forms the mechanism by which the interaction between S1 and the B-Oligomer is destabilized.