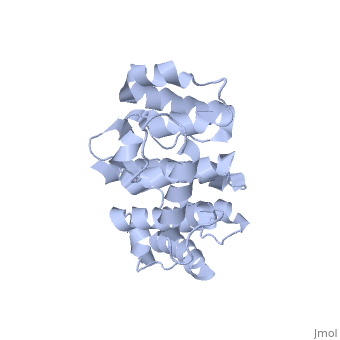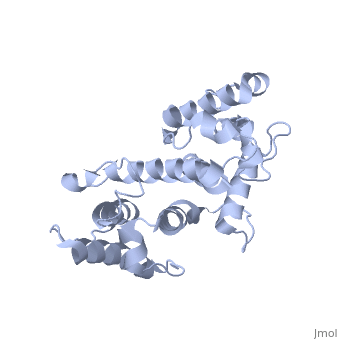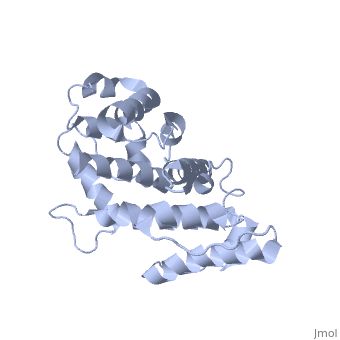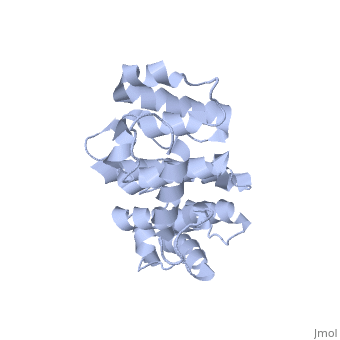
Plectin, a universal and functionally versatile cytolinker protein, can be divided in three main sections; a central coiled-coil rod domain, N and C-terminal globular region and exhibits a dumbbell like structure ([1]). C-terminal region is composed of 6 homologous repeating domains, and this region has a role in binding to intermediate filaments such as vimentin and cytokeratin (2). N-terminal globular region contains actin binding domain (ABD) comprising two calponin homology (CH) domains and N-terminal arm, which varies among isoforms (3).
- ↑ Foisner R, Wiche G. Structure and hydrodynamic properties of plectin molecules. J Mol Biol. 1987 Dec 5;198(3):515-31. PMID:3430617