Sandbox Reserved 344
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
| |||||||||
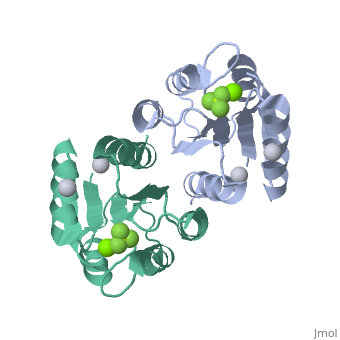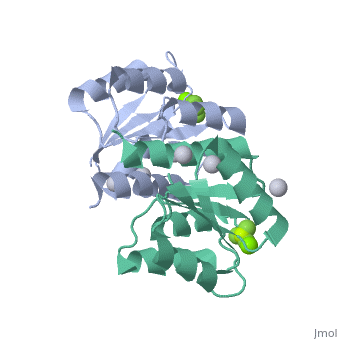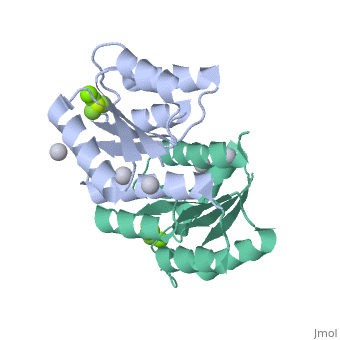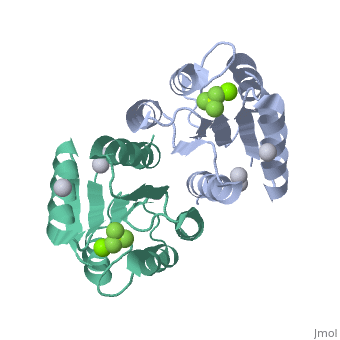
| 2pl1, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Non-Standard Residues: | |||||||||
| Gene: | phoP (Escherichia coli) | ||||||||
| Related: | 2pkx | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
IntroductionIntroduction
PhoP is a cytoplasmic response regulator from the two component system PhoP/PhoQ. This system responds mainly to changes in extracellular Mg2+ concentration and is an important part of the signal pathway leading to a coordinated cell response in gram-negative bacteria such as Escherichia coli and Salmonella enterica. PhoQ, located across the inner membrane, responds to low Mg2+ concentration by phosphorylating PhoP. Phosphorylated PhoP forms a homodimer and affects gene expression and other two component systems. Gene regulation is achieved by the increased affinity of the homodimer to the PhoP box, a tandem repeat promoter. The response is organism specific but generally involves survival in low Mg2+ environments and virulence. [1][2]
StructureStructure
PhoP consists of 2 domains, the regulatory domain and the C-terminal effector domain.[1]
Regulatory DomainRegulatory Domain
The regulatory domain consists of 5 α-helixes and 5 β-sheets. Twofold symmetry is achieved on the α-4 helix, β-5 sheet and α-5 helix face. The regulatory domain may be phosphorylated at a conserved aspartate by PhoQ, a histidine protein kinase. Phosphorylation of this aspartate stabilizes the homodimer.[1] The PhoP regulatory domain has intrinsic autophosphatase activity, allowing it to inactivate itself after a delay.
Un-activated formUn-activated form
- Under normal physiological conditions, unactivated PhoP occurs mainly as a monomer. At higher concentration unactivated PhoP has been shown to dimerize and act in a similar way to activated and dimerized PhoP. Many regulatory domains isolated from members of the OmpR/PhoB family and in their inactive form, crystalize in a form similar to their activated dimers.
Activated formActivated form
- Phosphorylation of the regulatory domain stabilizes dimer formation.
- BeF3-: Phosphoryl analog
- F1 to Mg2+
- F2 to Thr 79 (Correlated with Ala 80) -> F3?
- & BBone of Gly 53
- F3 to Lys 101 (salt bridge)
Effector domainEffector domain
The effector domain is activated when PhoP is in dimer form. There is no direct change in conformation conferred on the effector domain by the regulatory domain. The PhoP has a winged helix-turn-helix motif characteristic of the OmpR/PhoB family of response regulators.[3] This winged helix-turn-helix allows binding to DNA and regulation of transcription. Binding occurs at promoters with two repeats of the sequence (T/G)GTTTA, known as the PhoP box.[2]
FunctionFunction
Two component systems allow bacteria to respond to changes in their environment. These systems are found mainly in prokaryotes and a few eukaryotes.[4] The PhoP/PhoQ system, found specifically in gram-negative bacteria, react mainly to a drop in extracellular Mg2+. Since the magnesium concentration is typically lower inside the host cell compared to outside, this acts as a trigger to become virulent. Other functions activated by the PhoP/PhoQ system includes adaptation to low Mg2+ conditions, changes in cell wall, expression of proteases to protect against antimicrobial peptides and various other species specific responses. PhoP in Salmonella enterica regulates up to 40 proteins.[2] PhoP is not limited to direct regulation of gene expression. PhoP may regulate other two component systems at transcription, posttranscription and posttranslation too, in fact PhoP activates the expression of its own phoP gene, resulting in positive feedback and definite conversion to virulence. Further evidence to PhoP's role in response to the extracellular environment is given by evidence of sRNA regulation of the expression of the phoP gene. Envelope stress, though σE and the sRNA MicA affects expression of the PhoPQ system. [5]
Birck: 12486062 (winged helix...)
The PhoP/PhoQ system may also be found in non-cytoplasmic pathogens, such as Erwinia carotovora supsb. carotovora, a plant pathogen living in the intercellular fluid, or non-pathogenic bacteria. [2]
Importance of PhoPImportance of PhoP
PhoP/PhoQ plays in key role in certain bacteria becoming virulent. This makes it a promising target for vaccine and antimicrobial drug development.
ReferencesReferences
- ↑ 1.0 1.1 1.2 Bachhawat P, Stock AM. Crystal structures of the receiver domain of the response regulator PhoP from Escherichia coli in the absence and presence of the phosphoryl analog beryllofluoride. J Bacteriol. 2007 Aug;189(16):5987-95. Epub 2007 Jun 1. PMID:17545283 doi:10.1128/JB.00049-07
- ↑ 2.0 2.1 2.2 2.3 Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001 Mar;183(6):1835-42. PMID:11222580 doi:10.1128/JB.183.6.1835-1842.2001
- ↑ Gomez Barroso JA, Pereira H, Miranda M, Pereira C, Garratt RC, Aguilar CF. Protein preparation, crystallization and preliminary X-ray analysis of Trypanosoma cruzi nucleoside diphosphate kinase 1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 Jul 1;66(Pt, 7):862-5. Epub 2010 Jun 26. PMID:20606293 doi:10.1107/S1744309110013886
- ↑ Mack TR, Gao R, Stock AM. Probing the roles of the two different dimers mediated by the receiver domain of the response regulator PhoB. J Mol Biol. 2009 Jun 5;389(2):349-64. Epub 2009 Apr 14. PMID:19371748 doi:10.1016/j.jmb.2009.04.014
- ↑ Coornaert A, Lu A, Mandin P, Springer M, Gottesman S, Guillier M. MicA sRNA links the PhoP regulon to cell envelope stress. Mol Microbiol. 2010 Apr;76(2):467-79. Epub 2010 Mar 25. PMID:20345657 doi:10.1111/j.1365-2958.2010.07115.x