Jasper Lactate Final
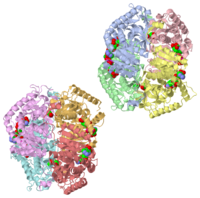
HUMAN MUSCLE L-LACTATE DEHYDROGENASE M CHAIN, TERNARY COMPLEX WITH NADH AND OXAMATEHUMAN MUSCLE L-LACTATE DEHYDROGENASE M CHAIN, TERNARY COMPLEX WITH NADH AND OXAMATE
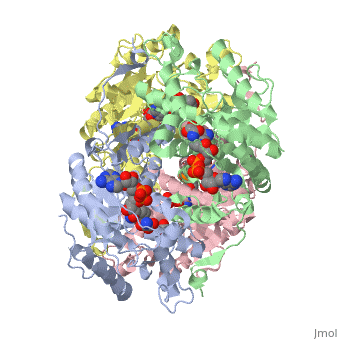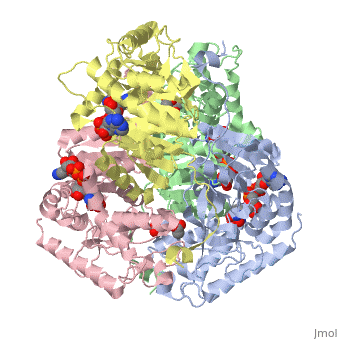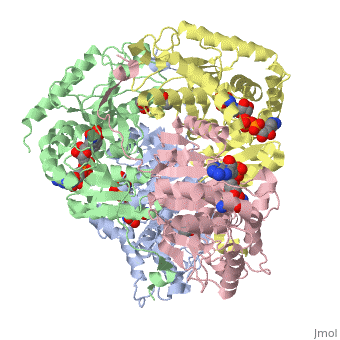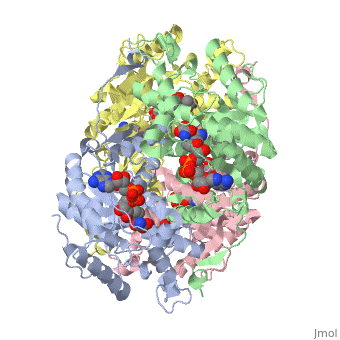
Lactate DehydrogenaseLactate Dehydrogenase
is an important enzyme in humans. It occurs in different regions of the body, each region having a unique conformation of different subunits. The Jmole image shown is that of LDH-5, the form found in skeleton muscle and the liver. LDH is a key enzyme in anaerobic respiration. Anaerobic Respiration is the conversion of pyruvate into lactate acid in the absence oxygen. This pathway is important to glycolysis in two main ways. The first is that if pyruvate were to build up glycoysis and thus the generation of ATP would slow. The second is anaerobic respiration allows for the regeneration of NAD+ from NADH. NAD+ is required when glyceraldehyde-3-phosphate dehydrogenase oxidizes glyceraldehyde-3-phosphate in glycolysis, which generates NADH. Lactate dehydrogenase is responsible for the anaerobic conversion of NADH to NAD+.

StructureStructure
LDH is a quaternary protein formed of the combination of two subunits, M and H (Muscle and Heart) into a structure of four of the subunits. The various combinations found in the human body are:
- (4H) Heart
- (3H1M) Reticuloendothelial
- (2H2M) Lungs
- (1H3M) Kidneys
- (4M) Muscle and Liver
The of LDH as shown here is comprised of 40% alpha helices and 23% beta sheets.(2) The SCOP data classifies this form of lactate dehydrogenase as mixed beta-alpha-beta, with mainly parallel beta sheets.
CatalysisCatalysis
Studies have shown that the reaction mechanism of LDH follows an ordered sequence. In order for lactate to be oxidized NADH must bind to the enzyme first followed by lactate. Several residues are involved in the binding of NADH, including and Tyr 85. Once the NADH is bound to the enzyme, it is then possible for lactate to bind. Lactate binds to the enzyme between the nicotinamide ring and . Transfer of a hydride ion then happens quickly in either direction giving a mixture of the two tertiary complexes, enzyme-NAD+-lactate and enzyme-NADH-pyruvate. Finally pyruvate dissociates from the enzyme followed by NAD+. The rate limiting step in this reaction is the rate of dissociation of NAD+. The same holds true in the reverse reaction that the coenzyme, NADH, must bind first before the substrate, pyruvate, can bind. The conversion of pyruvate to lactate with the subsequent regeneration of NAD+ is a very favorable(1).

ReferenceReference
1- http://www.bioc.aecom.yu.edu/labs/calllab/highlights/LDH.htm 2- http://www.cheric.org/ippage/e/ipdata/2004/05/file/e200405-701.pdf