Glyceraldehyde-3-Phosphate Dehydrogenase
Glyceraldehyde-3-Phosphate DehydrogenaseGlyceraldehyde-3-Phosphate Dehydrogenase
| |||||||||
| 3gpd, resolution 3.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Glyceraldehyde-3-phosphate dehydrogenase (phosphorylating), with EC number 1.2.1.12 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a very important enzyme in the production of energy. This enzyme catalyzes the sixth step in the process of breaking down glucose into energy, also known as glycolysis. Though this is its main function, GAPDH has been shown to perform other functions including transcription activation, initiation of apoptosis, and ER to Golgi apparatus vesicle transportation. However, this page will focus on GAPDH’s role in glysolysis.
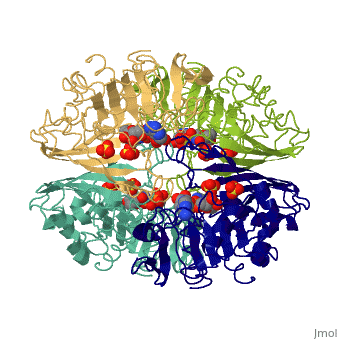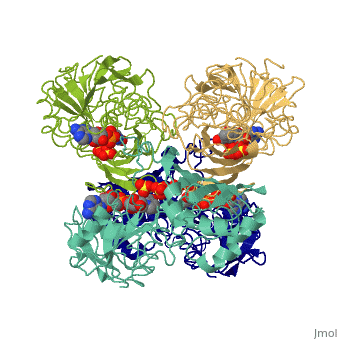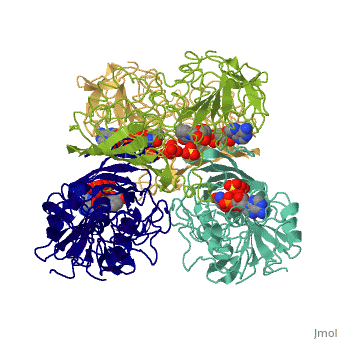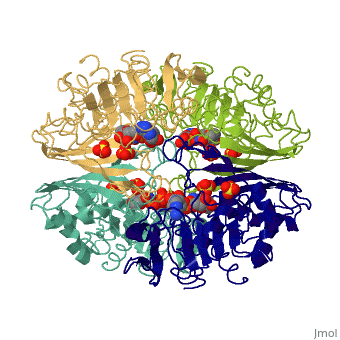
GAPDH most commonly exists as what looks to be a dimer. Interesting though, the two monomers of the enzyme are not exactly the same. While one side consists only of parallel and antiparallel beta-sheets, the other monomer is made up of both . This is consistent with the following SCOP information:
Class: Alpha and beta proteins (a/b)
Fold: NAD(P)-binding Rossmann-fold domains
Superfamily: NAD(P)-binding Rossmann-fold domains
Family: Glyceraldehyde-3-phosphate dehydrogenase-like, N-terminus domain
Protein: Glyceraldehyde-3-phosphate dehydrogenase
Species: Human
The specific reaction that GAPDH catalyzes is shown below:
The mechanism of the glycolysis reaction is fairly straight forward. After the aldehyde enters the , the sulfhydryl group from attacks the nucleophilic carbon to form a thiohemiacetal. This intermediate undergoes oxidation due to a hydride transfer to a nearby NAD+ forming a thioester. From here, a phosphate group enters and attacks the same carbonyl while at the same time it is separated from the cystine by the protonated group. This produces the desired 1,3-bisphosphoglycerate.