Succinyl-CoA synthetase
|
Succinyl-Coa synthetase catalyzes the reversible reaction of succinyl-CoA + NDP + Pi <-> succinate + CoA + NTP (where N is either adenosine or guanosine. It can be found in Escherichia coli.
StructureStructure
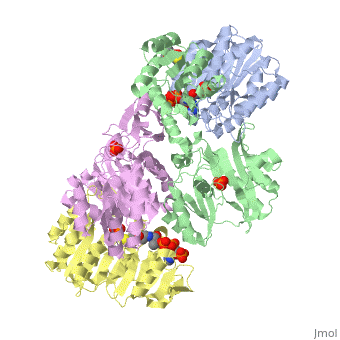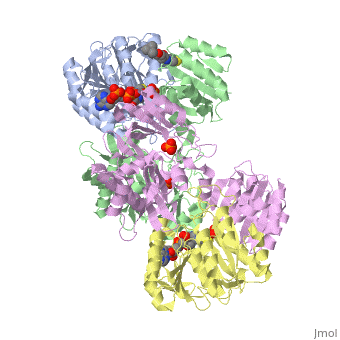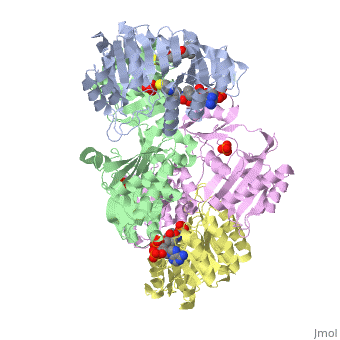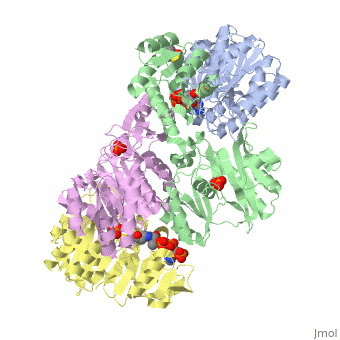
Succinyl-CoA synthetase is a tetramer with an active site on each dimer. This can be seen in the Jmol representation (the PDB code is 1CQJ). The two dimers are denoted alpha and beta. A phosphorylated histidine intermediate is responsible for the dephosphorylation of ATP and it is suspected that there are other active sites on the beta dimer that are responsible for the continued catalysis of the reaction.
Trypsin-BPTI complexTrypsin-BPTI complex
The trypsin backbone is shown in pink and the trypsin inhibitor, BPTI, in yellow (PDB code 2ptc). The residues [Ser195-His57-Asp102-Ser214] are shown in green, the disulfide bond between residues 14-38 is shown in yellow and the Lys 15 sidechain at the specificity site in pink.