Intrinsically Disordered Protein
Recently recognized as a distinct category of proteins [1][2][3]. Also known as intrinsically disordered proteins (IDP) or natively unfolded proteins. The term refers to proteins or to sequences within proteins that, under physiological conditions in-vitro, display physicochemical characteristics resembling those of random coils. Possessing little or no ordered structure with extended conformation and high intra-molecular flexibility, lacking of a tightly packed core.
Examples of IUPsExamples of IUPs
Examples cover a wide variety of cellular systems and it has been predicted that eukaryotes have more IUPs than other kingdoms [4].
IUPs play roles in processes such as:
- Cell signaling and cell cycle regulation, e.g. cyclin dependent kinase inhibitor p21Waf1/Cip1/Sdi1[5]
- Oncogene, e.g. P53 contains large unstructured regions in its native state[6]
- Assembly of cytoskeletal proteins, e.g. Tau protein[7]
- Membrane fusion and membrane transport, e.g. isolated components of the SNARE complex[8]
- DNA recognition molecules, e.g. the basic DNA-binding region of the leucine zipper protein, GCN4[9]
- Protein-RNA recognition, e.g. ribosomal proteins L11-C76[10]
- Amyloid formation, e.g. prion protein, N terminal part[13], NACP precursor of the non-Ab component of the amyloid plaque[14]
Protein disorder predictorsProtein disorder predictors
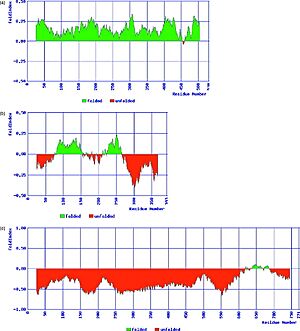 |  |
Led by the assumption that “since amino acid sequence determines 3-D structure, amino acid sequence should also determine lack of 3-D structure” [17] specific sequence features shared by IUPs have been evaluated and algorithms for their identification formulated.
IUPs occupy the low hydrophobicity/ high net-charge portion of charge-hydrophobicity phase space, which is the basis of the FoldIndex predictor .
Other predictors includes, DISOPRED, PONDR, IUPred and more.
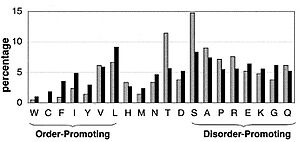
The low hydrophobicity and high net charge of naively unfolded proteins result in a difference in amino acid composition between them and naively folded proteins [18]. Compared to sequences of ordered proteins, disordered protein sequences are substantially depleted in I, L, V, W, F, Y, and C, which were therefore designated as “order promoting” amino acids, and enriched in E, K, R, G, Q, S, P, and A, which have been designated as “disorder promoting”. The under representation of hydrophobic amino acids in a protein diminishes one of the basic thermodynamic forces known to be important for protein folding, namely, the hydrophobic interaction. Because a hydrophobic core does not form, such proteins have large hydrodynamic dimensions.
Biological implications of IUPsBiological implications of IUPs
It was proposed that the unfolded nature of the IUPs provides them with advantages in recognition and binding. Although their large hydrodynamic dimensions slow down diffusion, their size provides a large target for initial molecular collisions, and the lack of rigid binding pockets permits multiple approach orientations for a binding partner, which may increase the probability of productive interactions [19][17]. In addition, IUPs allow molecular plasticity by adopting more than one conformation and binding diversity by binding to several proteins and thus many of the known hub proteins are IUPs. IUPs rapid turnover in the cell allow their tight regulation as many times needed in cell signaling and cell cycle.
Many IUPs undergo disorder-order transitionMany IUPs undergo disorder-order transition
Binding of natural ligands such as a variety of small molecules, substrates, cofactors, other proteins, nucleic acids or membranes induce folding of the unstructured protein. See the following two examples and also the page on Lac repressor
The human p27Kip1 kinase inhibitory domain [20]The human p27Kip1 kinase inhibitory domain [20]
The cyclin-dependent kinases (CDKs) have a central role in coordinating the eukaryotic cell division cycle. CDKs are controlled through several different processes involving the binding of activating cyclin subunits. Complexes of cyclins with CDKs play a central role in the control of the eukaryotic cell cycle. These complexes are inhibited by other proteins termed in general cyclin-CDK inhibitors (CKIs). One example of CKIs is p27Kip1. p27Kip1 is an IUP and it binds to phosphorylated in interacting with both and . On cyclin A, it binds in a groove formed by conserved cyclin box residues. On CDK2, it binds and rearranges the amino-terminal lobe and also inserts into the catalytic cleft, mimicking ATP. [[1]]
|
The transcriptional activator GCN4 [21]The transcriptional activator GCN4 [21]
The yeast transcriptional activator GCN4 belongs to a large family of eukaryotic transcription factors including Fos, Jun and CREB. All family members have a DNA recognition motif consists of a coiled-coil dimerization element, the leucine-zipper, and an adjoining basic region, which mediates DNA binding. This basic region is largely unstructured in the absence of DNA, addition of DNA containing a GCN4 binding site induce the transition of this region from unstructured to α-helical.
ReferencesReferences
- ↑ Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999 Oct 22;293(2):321-31. PMID:10550212 doi:10.1006/jmbi.1999.3110
- ↑ Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z. Intrinsically disordered protein. J Mol Graph Model. 2001;19(1):26-59. PMID:11381529
- ↑ Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002 Jan;269(1):2-12. PMID:11784292
- ↑ Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Inform. 2000;11:161-71. PMID:11700597
- ↑ Kriwacki RW, Hengst L, Tennant L, Reed SI, Wright PE. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11504-9. PMID:8876165
- ↑ Bell S, Klein C, Muller L, Hansen S, Buchner J. p53 contains large unstructured regions in its native state. J Mol Biol. 2002 Oct 4;322(5):917-27. PMID:12367518
- ↑ Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290-7. PMID:7929085
- ↑ Fiebig KM, Rice LM, Pollock E, Brunger AT. Folding intermediates of SNARE complex assembly. Nat Struct Biol. 1999 Feb;6(2):117-23. PMID:10048921 doi:10.1038/5803
- ↑ Weiss MA, Ellenberger T, Wobbe CR, Lee JP, Harrison SC, Struhl K. Folding transition in the DNA-binding domain of GCN4 on specific binding to DNA. Nature. 1990 Oct 11;347(6293):575-8. PMID:2145515 doi:http://dx.doi.org/10.1038/347575a0
- ↑ Markus MA, Hinck AP, Huang S, Draper DE, Torchia DA. High resolution solution structure of ribosomal protein L11-C76, a helical protein with a flexible loop that becomes structured upon binding to RNA. Nat Struct Biol. 1997 Jan;4(1):70-7. PMID:8989327
- ↑ Schmitz ML, dos Santos Silva MA, Altmann H, Czisch M, Holak TA, Baeuerle PA. Structural and functional analysis of the NF-kappa B p65 C terminus. An acidic and modular transactivation domain with the potential to adopt an alpha-helical conformation. J Biol Chem. 1994 Oct 14;269(41):25613-20. PMID:7929265
- ↑ Baskakov IV, Kumar R, Srinivasan G, Ji YS, Bolen DW, Thompson EB. Trimethylamine N-oxide-induced cooperative folding of an intrinsically unfolded transcription-activating fragment of human glucocorticoid receptor. J Biol Chem. 1999 Apr 16;274(16):10693-6. PMID:10196139
- ↑ Donne DG, Viles JH, Groth D, Mehlhorn I, James TL, Cohen FE, Prusiner SB, Wright PE, Dyson HJ. Structure of the recombinant full-length hamster prion protein PrP(29-231): the N terminus is highly flexible. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13452-7. PMID:9391046
- ↑ Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996 Oct 29;35(43):13709-15. PMID:8901511 doi:10.1021/bi961799n
- ↑ Prilusky J, Felder CE, Zeev-Ben-Mordehai T, Rydberg EH, Man O, Beckmann JS, Silman I, Sussman JL. FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics. 2005 Aug 15;21(16):3435-8. Epub 2005 Jun 14. PMID:15955783 doi:http://dx.doi.org/10.1093/bioinformatics/bti537
- ↑ Zeev-Ben-Mordehai T, Rydberg EH, Solomon A, Toker L, Auld VJ, Silman I, Botti S, Sussman JL. The intracellular domain of the Drosophila cholinesterase-like neural adhesion protein, gliotactin, is natively unfolded. Proteins. 2003 Nov 15;53(3):758-67. PMID:14579366 doi:10.1002/prot.10471
- ↑ 17.0 17.1 Dunker AK, Obradovic Z. The protein trinity--linking function and disorder. Nat Biotechnol. 2001 Sep;19(9):805-6. PMID:11533628 doi:10.1038/nbt0901-805
- ↑ Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins. 2001 Jan 1;42(1):38-48. PMID:11093259
- ↑ Denning DP, Uversky V, Patel SS, Fink AL, Rexach M. The Saccharomyces cerevisiae nucleoporin Nup2p is a natively unfolded protein. J Biol Chem. 2002 Sep 6;277(36):33447-55. Epub 2002 Jun 13. PMID:12065587 doi:10.1074/jbc.M203499200
- ↑ Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996 Jul 25;382(6589):325-31. PMID:8684460 doi:10.1038/382325a0
- ↑ Konig P, Richmond TJ. The X-ray structure of the GCN4-bZIP bound to ATF/CREB site DNA shows the complex depends on DNA flexibility. J Mol Biol. 1993 Sep 5;233(1):139-54. PMID:8377181 doi:http://dx.doi.org/10.1006/jmbi.1993.1490