4ald
|
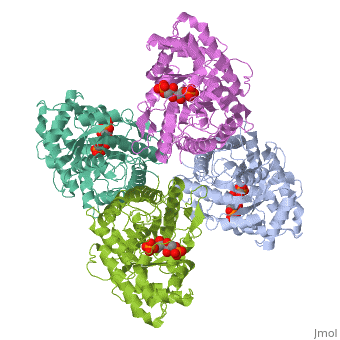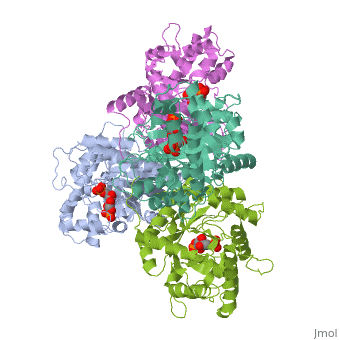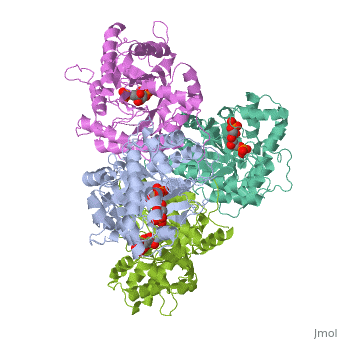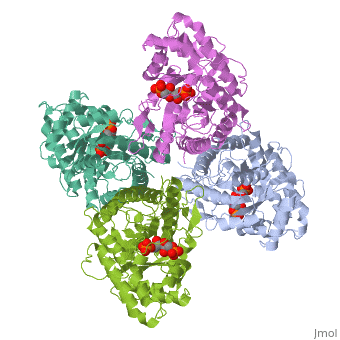
HUMAN MUSCLE FRUCTOSE 1,6-BISPHOSPHATE ALDOLASE COMPLEXED WITH FRUCTOSE 1,6-BISPHOSPHATE
OverviewOverview
Fructose 1,6-bisphosphate aldolase catalyzes the reversible cleavage of, fructose 1,6-bisphosphate and fructose 1-phosphate to dihydroxyacetone, phosphate and either glyceraldehyde 3-phosphate or glyceraldehyde, respectively. Catalysis involves the formation of a Schiff's base, intermediate formed at the epsilon-amino group of Lys229. The existing, apo-enzyme structure was refined using the crystallographic free-R-factor, and maximum likelihood methods that have been shown to give improved, structural results that are less subject to model bias. Crystals were also, soaked with the natural substrate (fructose 1,6-bisphosphate), and the, crystal structure of this complex has been determined to 2.8 A. The apo, structure differs from the previous Brookhaven-deposited structure (1ald), in the ... [(full description)]
About this StructureAbout this Structure
4ALD is a [Single protein] structure of sequence from [Homo sapiens] with 2FP as [ligand]. The following page contains interesting information on the relation of 4ALD with [The Glycolytic Enzymes]. Active as [Lyase], with EC number [4.1.2.13]. Structure known Active Site: SBL. Full crystallographic information is available from [OCA].
ReferenceReference
Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications., Dalby A, Dauter Z, Littlechild JA, Protein Sci. 1999 Feb;8(2):291-7. PMID:10048322
Page seeded by OCA on Tue Oct 30 08:36:53 2007