SARS-CoV-2 spike protein fusion transformation
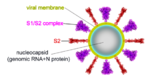
The spike protein of SARS-CoV-2 plays a central role in coronavirus attachment to the ACE2 receptor on host cells, and in getting the RNA genome of the virus into the host cell via fusion of the virus and host cell membranes, initiating infection. You may find it helpful to read SARS-CoV-2 spike protein priming by furin before continuing with the article below.
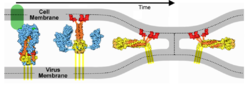
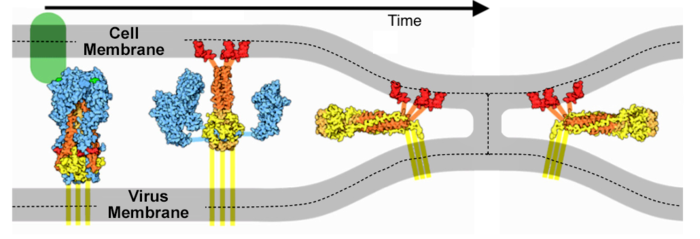
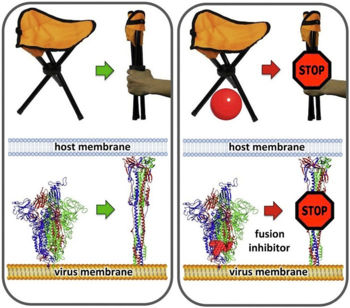
IntroductionSARS-CoV-2 spike protein undergoes a dramatic conformational rearrangement () that plays a central role in fusing the coronavirus membrane with the host cell membrane[1]. Similar conformational transformations have been observed for the spike protein of SARS-CoV[2] and mouse hepatitis virus[3], among others. These rearrangements also have much in common with the membrane fusion mechansism of influenza hemagglutinin[4][5]. The molecular scenes in this article are based on the cryo-EM pre- and post-fusion structures of SARS-CoV-2 spike protein reported July, 2020, by Cai, Zhang and coworkers with the group of Bing Chen[1].
Pre-Fusion Structure
The is a homo-trimer, each chain having a mature length of 1,261 amino acids. This pre-fusion cryo-EM structure 6xr8 is complete except for 110 residues of the C-terminus (narrow end), comprising 48 residues of the stem (heptad repeat 2 domain), a 23-residue trans-membrane domain, and a 39 residue cytoplasmic domain. Domainsas in Cai, Zhang et al. [1] (lengths):
ActivationA protease, typically furin, cuts the chains at the position marked with Pink balls[6], but the assembly, now six chains, remains intact. The cut may facilitate extending one receptor binding domain to engage the ACE2 receptor. This "priming" cut divides each chain of protein S into an N-terminal S1 receptor-binding fragment, and a C-terminal S2 fusion fragment[1]. , will later separate. Binding to ACE2 and a second proteolytic cut are believed to trigger release of S1[1], after which through the stem and transmembrane domains (absent in this model) that extend from the red balls. Limitations of ModelsNext, a dramatic conformational transformation occurs that triggers membrane fusion. We will compare the pre-fusion cryo-EM model (6xr8) with the post-fusion model (6xra). However, these two models have only two protein segments in common, sequence ranges 703-770 and 912-1162 (319 residues or 54% of the 588-residue S2 fusion fragment, 686-1273). The parts of the pre-fusion model . Thus, the pre- to post-fusion comparison will involve . FusionFusion of the virus and host cell membranes seems to happen when the spike protein brings the virus membrane (near the red balls) very close to the host membrane, after the host membrane is attached to the fusion peptide (near the blue balls).
Remember that a morph is intended to help you compare two structures. This morph does NOT portray a realistic transition pathway.
An [7] between the two conformations shows how some secondary structure is retained while structural elements change orientation and position substantially. USe the button above to start and pause the morph.
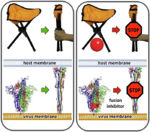
Membrane Fusion SchematicNow that you understand the various movements taking place, you will better appreciate . The membrane fusion hypothesis may be clarified by this schematic. It is for influenza virus but the principles are the same.
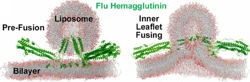
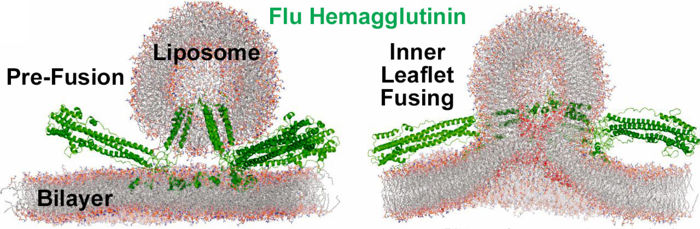
Molecular Dynamics Simulation of Membrane FusionMolecular dynamics simulations are consistent with this hypothesis. Below is shown a liposome containing influenza hemagglutinin fusing with a lipid bilayer in a molecular dynamics simulation[5].
GlycosylationBoth the , and the have extensive N-linked glycosylation (red and gray, exaggerated in size because the cryo-EM can likely resolve only the protein proximal portions). No glycans were observed on the receptor-binding surface. Glycosylation may protect the spike protein from antibodies and other damage from the virion environment[1]. Unexpected FindingsSpontaneous Fusion Transformation
The fusion transformation, including loss of S1, occurred in a substantial portion of the full-length spike protein purified in mild detergent, in the absence of host cells or ACE2 receptors. Cai, Zhang and coworkers[1] note that post-fusion S2 conformations have been observed multiple times in electron micrographs by other researchers. They suggest that virions normally contain a mixture of pre-fusion full-length, and post-fusion S2 fragment conformations, and that the post-fusion S2 spike proteins may help to protect the pre-fusion forms, and possibly may induce non-neutralizing antibody responses. More Compact StructurePrevious studies of soluble extracellular portions of the spike protein have utilized a double proline mutation (K986P, V987P) that stabilizes the pre-fusion conformation[1][9]. K986P appears to eliminate a salt bridge, causing an unsatisfied internal charge[1]. The wild type salt bridge may contribute to the more compact and better resolved structure observed by Cai, Zhang and coworkers[1] using the full-length wild type protein. Preventing Fusion with Drugs
SARS-CoV-2 spike protein has two interior cavities: (i) (6zgi) and then (ii)
The larger interior cavity opens to the surface when one receptor binding site is extended. The smaller interior cavity seems crucial to fusion function. Many fusion inhibiting drugs have been found to bind within a similar smaller cavity in related proteins, and escape mutations have clustered in or near this cavity[10]. Using virtual screening simulations with a cryo-EM model of SARS-CoV-2 spike protein (6vsb), Romeo, Iacovelli & Falconi[10] identified available drugs, already approved or in clinical trial, that seem likely to block the fusion transformation by binding in the smaller cavity.
Scroll below for related pages, Powerpoint-ready animations, Methods, References & Notes.
|
| |||||||||||||||||||||||||||||
Animations for SlidesAnimations for Slides
These animations are ready to be dropped into presentation slides (Powerpoint, Google Slides, Libre Office Impress, etc.). As above, the red balls are close to the virus envelope membrane, and the blue balls are close to the host cell membrane. As above, these are linear interpolation morphs from 6xr8 to 6xra.
Solid Fusion TransformationSolid Fusion Transformation
Download (right click, save link as) 500 px wide solid animation. This shows alpha carbons only, exaggerated in size to 6.2 Å diameter to make a solid object. Please credit Proteopedia.Org in accord with our license, and Cai, Zhang and coworkers[1] for their cryo-EM structures.
Main Chain Fusion TransformationMain Chain Fusion Transformation
Download (right click, save link as) 500 px wide main chain animation. This shows a smoothed main chain (backbone) trace. Please credit Proteopedia.Org in accord with our license, and Cai, Zhang and coworkers[1] for their cryo-EM structures.
Alternate Pathway for TransformationAlternate Pathway for Transformation
Given that a morph is intended to help you compare two structures and does not portray a realistic transition pathway, there are many pathways we can imagine. Below is another one that has structural elements swing away from the core structure in a hinge motion as they change conformation. Another choice in this morph is to have different timings for the distinct changes to be able to follow them one at a time.
In the morph at full resolution, you can see a large cavity disappearing, the beta sheets acquiring an additional strand from a different subunit (subunits shown in green, blue and red tints), and changes in the core helix bundle along with the formation of the coiled-coil protrusion made of three helices.
Download (right click, save link as) 720 px wide main chain animation. This shows a smoothed main chain (backbone) trace. Please credit Proteopedia.Org in accord with our license, and Cai, Zhang and coworkers[1] for their cryo-EM structures.
Download (right click, save link as) 720 px wide main chain animation. This shows C-alpha atoms as large spheres. Please credit Proteopedia.Org in accord with our license, and Cai, Zhang and coworkers[1] for their cryo-EM structures.
Pre-Fusion Spike ProteinPre-Fusion Spike Protein
These show SARS-CoV-2 spike protein 6xr8 colored by domain (see color key above). The receptor binding domains are at the top. The bottom extends through parts missing in this model: stem, transmembrane domain, cytoplasmic domain. Pink balls mark the furin cleavage sites that separate S1 from S2. S1 is translucent in the movie at right. Please credit Proteopedia.Org in accord with our license, and Cai, Zhang and coworkers[1] for their cryo-EM structures.
| DOWNLOAD | DOWNLOAD |
Other Animations?Other Animations?
If you would like a presentation-ready animation of something else, feel free to contact us.
See AlsoSee Also
- SARS-CoV-2 spike protein priming by furin - the step prior to membrane fusion.
- SARS-CoV-2 spike protein mutations suspected to increase transmission rates.
- SARS-CoV-2 protein S
- Spike protein
- Coronavirus Disease 2019 (COVID-19)
- Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up about 6vsb
- Jmol/Cavities pockets and tunnels
- Cavity programs
MethodsMethods
MorphingMorphing
The pre-fusion structure 6xr8 was morphed to the post-fusion structure 6xra by linear interpolation, requesting 14 intermediate frames (16 total), using the server provided by Karsten Theis after another method[12] gave unsatisfactory results. To avoid artifactual movement in the morph, prior to morphing, two changes were required in 6xra: (i) the names of chains B and C needed to be swapped (done with SwissPDBViewer), and (ii) the structure needed to be rotated +120° around the Z axis (Jmol "rotateselected" command). The morph was an 11 MB file, which took 25 sec to load into JSmol. Each script took a minimum of 8 sec to complete. To reduce both the bulk of this file and the processing times for JSmol, the alpha carbons were extracted (along with the MODEL and ENDMDL records) by deleting all other lines in the PDB file[13]. The resulting 16 model morph PDB file is File:Morf-6xr8-6xra-theis-cao.pdb.
Interior CavitiesInterior Cavities
6zgi was loaded into the Jmol Java application (~11-fold faster than JSmol), and rendered as translucent backbone, each chain a different pastel color. The command
- isosurface minset 100 interior cavity 3.0 10.0
was executed (~45 sec). The numeric parameters in that command were determined by trial and error (see Jmol/Cavities pockets and tunnels). The cavity surface data were saved as Jmol voxel data, and uploaded to Proteopedia as File:6zgi-cavities.jvxl. The button above reads that file, rather than re-calculating the cavities, in order to display the interior cavity surface data much more quickly. See also Jmol/Cavities pockets and tunnels.
AcknowledgementAcknowledgement
Eric Martz thanks Deborah Spitz for a critique that improved this article.
References and NotesReferences and Notes
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM Jr, Rawson S, Rits-Volloch S, Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020 Jul 21. pii: science.abd4251. doi: 10.1126/science.abd4251. PMID:32694201 doi:http://dx.doi.org/10.1126/science.abd4251
- ↑ Fan X, Cao D, Kong L, Zhang X. Cryo-EM analysis of the post-fusion structure of the SARS-CoV spike glycoprotein. Nat Commun. 2020 Jul 17;11(1):3618. doi: 10.1038/s41467-020-17371-6. PMID:32681106 doi:http://dx.doi.org/10.1038/s41467-020-17371-6
- ↑ Walls AC, Tortorici MA, Snijder J, Xiong X, Bosch BJ, Rey FA, Veesler D. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci U S A. 2017 Oct 17;114(42):11157-11162. doi:, 10.1073/pnas.1708727114. Epub 2017 Oct 3. PMID:29073020 doi:http://dx.doi.org/10.1073/pnas.1708727114
- ↑ 4.0 4.1 Hamilton BS, Whittaker GR, Daniel S. Influenza virus-mediated membrane fusion: determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses. 2012 Jul;4(7):1144-68. doi: 10.3390/v4071144. Epub 2012 Jul 24. PMID:22852045 doi:http://dx.doi.org/10.3390/v4071144
- ↑ 5.0 5.1 5.2 Pabis A, Rawle RJ, Kasson PM. Influenza hemagglutinin drives viral entry via two sequential intramembrane mechanisms. Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7200-7207. doi:, 10.1073/pnas.1914188117. Epub 2020 Mar 18. PMID:32188780 doi:http://dx.doi.org/10.1073/pnas.1914188117
- ↑ 6.0 6.1 According to Cai, Zhang et al. (their Fig. 4), the initial cut by furin occurs between Arg685 and Ser686 at * in the sequence PRRAR*SVASQ. Thus, S1 is 13-685 (length 673, excluding a 12 residue signal sequence), or 53% of the original chain, leaving S2 as 686-1273, length 588, 47%.
- ↑ The Storymorph Jmol scripts were used to create the interpolation shown in the morph. [1] available on Proteopedia
- ↑ Permission obtained from Gary R. Whittaker August 6, 2020. Permission obtained from David Goodsell August 5, 2020.
- ↑ Wrobel AG, Benton DJ, Xu P, Roustan C, Martin SR, Rosenthal PB, Skehel JJ, Gamblin SJ. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat Struct Mol Biol. 2020 Jul 9. pii: 10.1038/s41594-020-0468-7. doi:, 10.1038/s41594-020-0468-7. PMID:32647346 doi:http://dx.doi.org/10.1038/s41594-020-0468-7
- ↑ 10.0 10.1 10.2 Romeo A, Iacovelli F, Falconi M. Targeting the SARS-CoV-2 spike glycoprotein prefusion conformation: virtual screening and molecular dynamics simulations applied to the identification of potential fusion inhibitors. Virus Res. 2020 Jun 18;286:198068. doi: 10.1016/j.virusres.2020.198068. PMID:32565126 doi:http://dx.doi.org/10.1016/j.virusres.2020.198068
- ↑ This quote is from the PubMedCentral version of the Romeo paper, after you click License Information: "Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active."
- ↑ Proteopedia's PyMOL morph server was used in both RigiMOL and linear modes, all atoms or only alpha carbon atoms. Rendering these as backbones or traces by Jmol gave broken lines. The reason for backbone breaking was not investigated further.
- ↑ Selecting *.ca in the Jmol Java application and saving a PDB file produced a PDB file with numerous errors. The desired result was obtained with this command in macOS Terminal: sed -e /REMARK/d -e /HETATM/d -e /^ATOM\ \ [\ 0-9][0-9][0-9][0-9][0-9]\ \ [CONS][\ B-Z].*$/d <original.pdb >product.pdb.