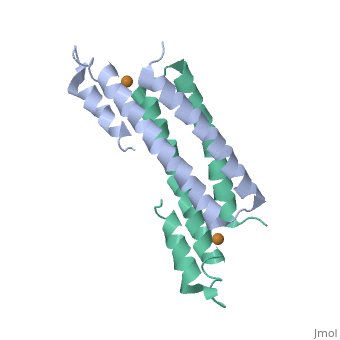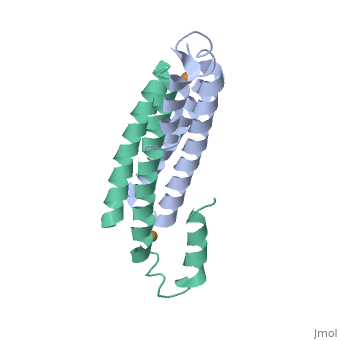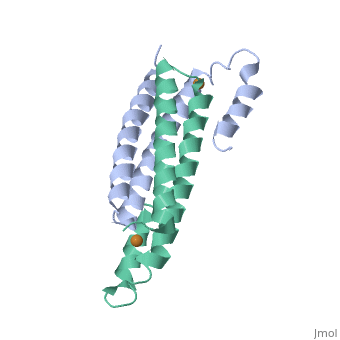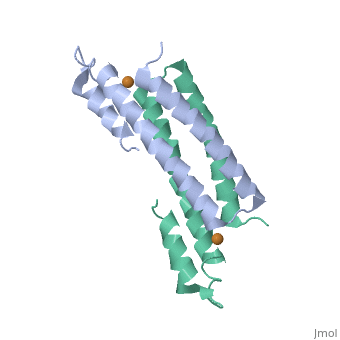
2hh7
Crystal Structure of Cu(I) bound CsoR from Mycobacterium tuberculosis.
| |||||||
| , resolution 2.55Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
OverviewOverview
Copper is an essential element that becomes highly cytotoxic when concentrations exceed the capacity of cells to sequester the ion. Here, we identify a new copper-specific repressor (CsoR) of a copper-sensitive operon (cso) in Mycobacterium tuberculosis (Mtb) that is representative of a large, previously uncharacterized family of proteins (DUF156). Electronic and X-ray absorption spectroscopies reveal that CsoR binds a single-monomer mole equivalent of Cu(I) to form a trigonally coordinated (S(2)N) Cu(I) complex. The 2.6-A crystal structure of copper-loaded CsoR shows a homodimeric antiparallel four-helix bundle architecture that represents a novel DNA-binding fold. The Cu(I) is coordinated by Cys36, Cys65' and His61' in a subunit bridging site. Cu(I) binding negatively regulates the binding of CsoR to a DNA fragment encompassing the operator-promoter region of the Mtb cso operon; this results in derepression of the operon in Mtb and the heterologous host Mycobacterium smegmatis. Substitution of Cys36 or His61 with alanine abolishes Cu(I)- and CsoR-dependent regulation in vivo and in vitro. Potential roles of CsoR in Mtb pathogenesis are discussed.
About this StructureAbout this Structure
2HH7 is a Single protein structure of sequence from Mycobacterium tuberculosis. Full crystallographic information is available from OCA.
ReferenceReference
CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator., Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP, Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269
Page seeded by OCA on Thu Mar 20 17:17:02 2008