Bam complex
Jump to navigation
Jump to search
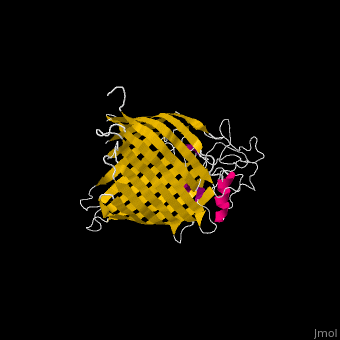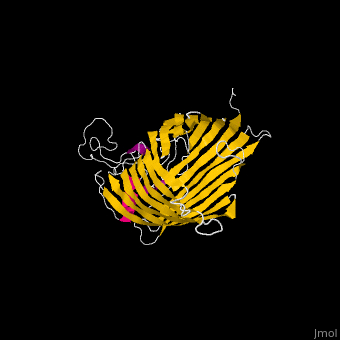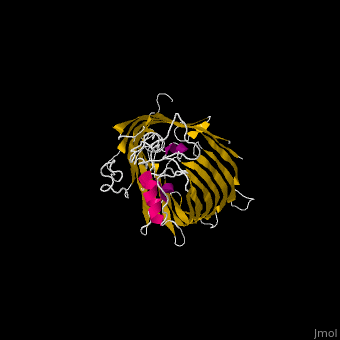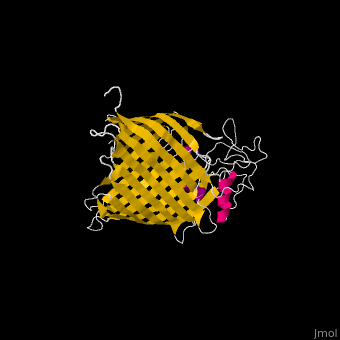
FunctionThe Bam (β-Barrel Assembly Machinery) drives the assembly of β-barrel proteins into the outer membrane of gram-negative bacteria.[1] Structural highlightsThe complex is composed of five subunits: BamA, BamB, BamC, BamD and BamE. Outer membrane β-barrel proteins assembly is dependent on Bam in various organisms. BamB,C,D,E bind to the N-terminal of BamA.
3D Structures of Bam complex
|
| ||||||||||
ReferencesReferences
- ↑ Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009 Mar;7(3):206-14. doi: 10.1038/nrmicro2069. Epub 2009 Feb , 2. PMID:19182809 doi:http://dx.doi.org/10.1038/nrmicro2069