Lotem haleva/test page
Your Heading Here (maybe something like 'Structure')Your Heading Here (maybe something like 'Structure')
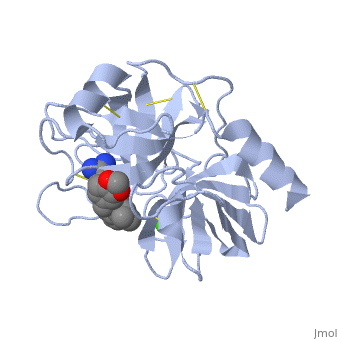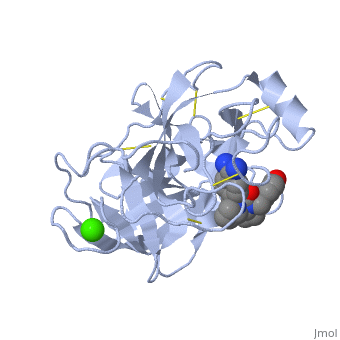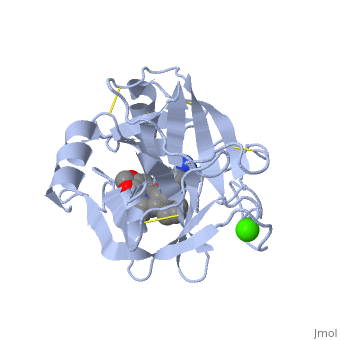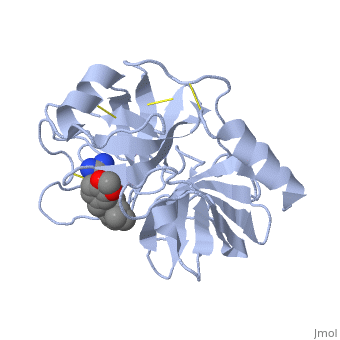
This is a default text for your page Lotem haleva/test page. Click above on edit this page to modify. Be careful with the < and > signs. is a serine protease, found in the digestive system of many vertebrates, where it hydrolyses proteins[1]. Trypsin is produced in the pancreas as the inactive protease trypsinogen. Trypsin Break peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when either is followed by proline. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinisation, and proteins that have been digested/treated with trypsin are said to have been trypsinized. FunctionIn the duodenum, trypsin catalyzes the hydrolysis of peptide bonds, breaking down proteins into smaller peptides. Trypsin contains and beta sheets.
MechanismThe enzymatic mechanism is similar to that of other serine proteases. These enzymes contain a catalytic triad consisting of . These three residues form a charge relay that serves to make the active site serine nucleophilic. Trypsin contains an "oxyanion hole" formed by the backbone amide hydrogen atoms of , which serves to stabilize the developing negative charge on the carbonyl oxygen atom of the cleaved amides. The located in the catalytic pocket (S1) of trypsin is responsible for attracting and stabilizing positively charged lysine and/or arginine, and is, thus, responsible for the specificity of the enzyme. RelevanceStructural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ Rawlings ND, Barrett AJ. Families of serine peptidases. Methods Enzymol. 1994;244:19-61. doi: 10.1016/0076-6879(94)44004-2. PMID:7845208 doi:http://dx.doi.org/10.1016/0076-6879(94)44004-2