1rop
STRUCTURE OF THE COL*E1 ROP PROTEIN AT 1.7 ANGSTROMS RESOLUTIONSTRUCTURE OF THE COL*E1 ROP PROTEIN AT 1.7 ANGSTROMS RESOLUTION
Structural highlights
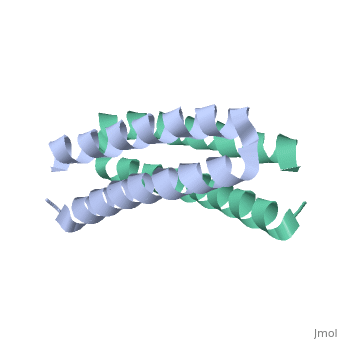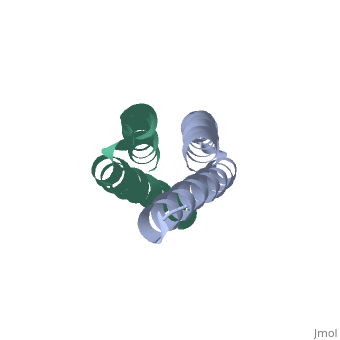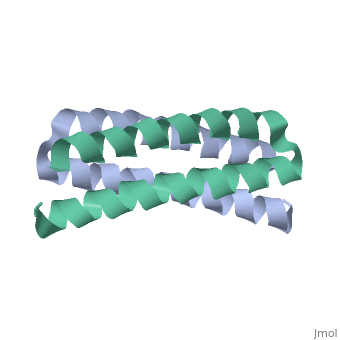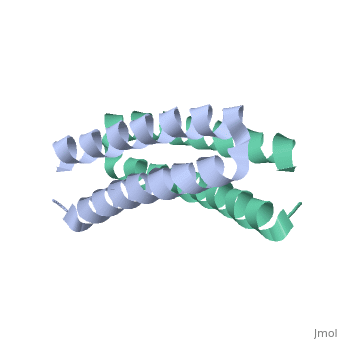
Function[ROP_ECOLX] Regulates plasmid DNA replication by modulating the initiation of transcription of the primer RNA precursor. Processing of the precursor of the primer, RNAII, is inhibited by hydrogen bonding of RNAII to its complementary sequence in RNAI. ROP increases the affinity of RNAI for RNAII and thus decreases the rate of replication initiation events. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedStructural details of the Rop protein from plasmid ColE1 are presented, with a description of the X-ray crystal structure determination and refinement at a nominal resolution of 1.7 A. The 63 amino acid protein is a dimer. Each monomer consists almost entirely of two alpha helices, the whole molecule forming a highly regular four-alpha-helix bundle. This may be approximated by a four-stranded rope with a radius of 7.0 A, a left-handed helical twist and a pitch of 172.5 A. The packing constraints for this novel type of coiled-coil structure are given. The protein acts in the control of plasmid replication via regulation of an RNA-RNA interaction in a manner not yet understood in atomic detail. Structure of the ColE1 rop protein at 1.7 A resolution.,Banner DW, Kokkinidis M, Tsernoglou D J Mol Biol. 1987 Aug 5;196(3):657-75. PMID:3681971[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||