3a0b
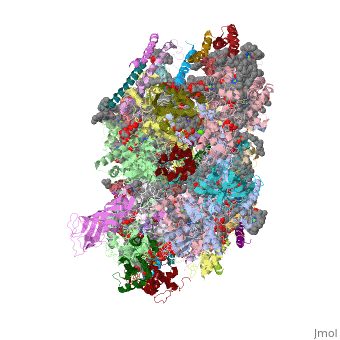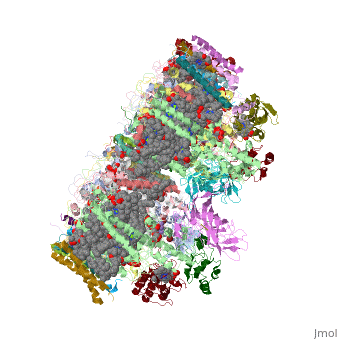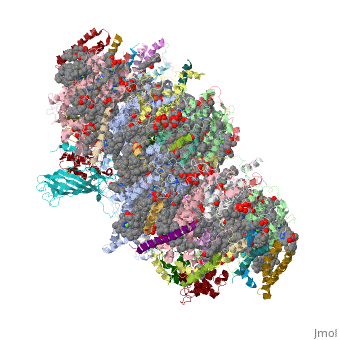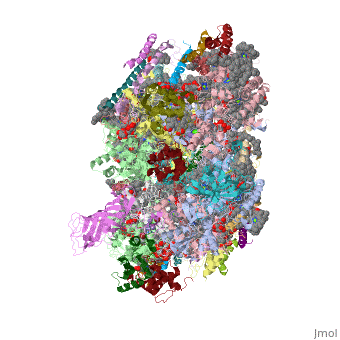
Crystal structure of Br-substituted Photosystem II complexCrystal structure of Br-substituted Photosystem II complex
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe chloride ion, Cl(-), is an essential cofactor for oxygen evolution of photosystem II (PSII) and is closely associated with the Mn(4)Ca cluster. Its detailed location and function have not been identified, however. We substituted Cl(-) with a bromide ion (Br(-)) or an iodide ion (I(-)) in PSII and analyzed the crystal structures of PSII with Br(-) and I(-) substitutions. Substitution of Cl(-) with Br(-) did not inhibit oxygen evolution, whereas substitution of Cl(-) with I(-) completely inhibited oxygen evolution, indicating the efficient replacement of Cl(-) by I(-). PSII with Br(-) and I(-) substitutions were crystallized, and their structures were analyzed. The results showed that there are 2 anion-binding sites in each PSII monomer; they are located on 2 sides of the Mn(4)Ca cluster at equal distances from the metal cluster. Anion-binding site 1 is close to the main chain of D1-Glu-333, and site 2 is close to the main chain of CP43-Glu-354; these 2 residues are coordinated directly with the Mn(4)Ca cluster. In addition, site 1 is located in the entrance of a proton exit channel. These results indicate that these 2 Cl(-) anions are required to maintain the coordination structure of the Mn(4)Ca cluster as well as the proposed proton channel, thereby keeping the oxygen-evolving complex fully active. Location of chloride and its possible functions in oxygen-evolving photosystem II revealed by X-ray crystallography.,Kawakami K, Umena Y, Kamiya N, Shen JR Proc Natl Acad Sci U S A. 2009 May 26;106(21):8567-72. Epub 2009 May 11. PMID:19433803[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||||