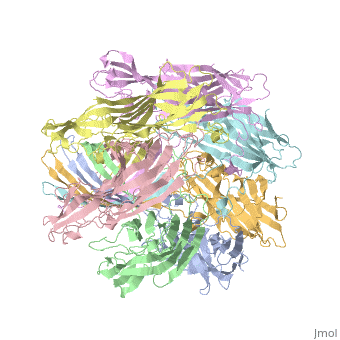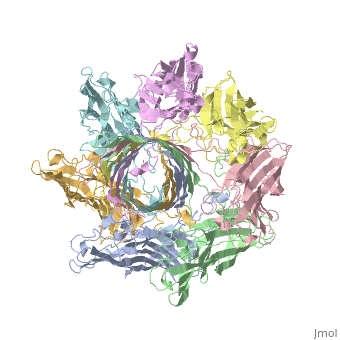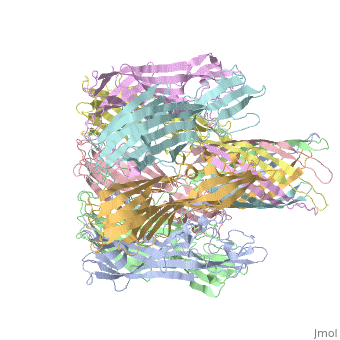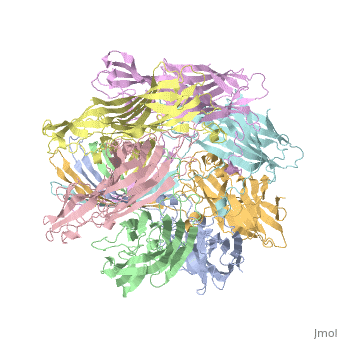7ahl
ALPHA-HEMOLYSIN FROM STAPHYLOCOCCUS AUREUSALPHA-HEMOLYSIN FROM STAPHYLOCOCCUS AUREUS
Structural highlights
Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe structure of the Staphylococcus aureus alpha-hemolysin pore has been determined to 1.9 A resolution. Contained within the mushroom-shaped homo-oligomeric heptamer is a solvent-filled channel, 100 A in length, that runs along the sevenfold axis and ranges from 14 A to 46 A in diameter. The lytic, transmembrane domain comprises the lower half of a 14-strand antiparallel beta barrel, to which each protomer contributes two beta strands, each 65 A long. The interior of the beta barrel is primarily hydrophilic, and the exterior has a hydrophobic belt 28 A wide. The structure proves the heptameric subunit stoichiometry of the alpha-hemolysin oligomer, shows that a glycine-rich and solvent-exposed region of a water-soluble protein can self-assemble to form a transmembrane pore of defined structure, and provides insight into the principles of membrane interaction and transport activity of beta barrel pore-forming toxins. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore.,Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE Science. 1996 Dec 13;274(5294):1859-66. PMID:8943190[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||