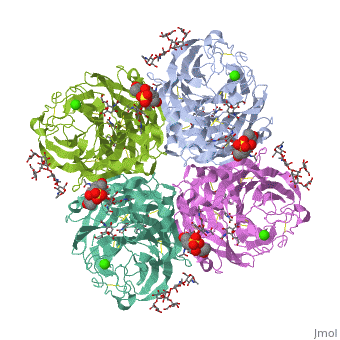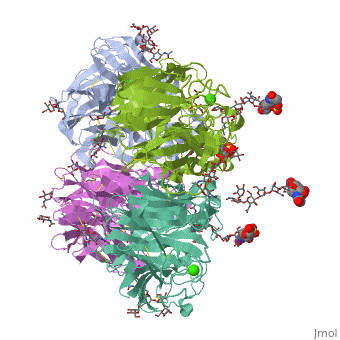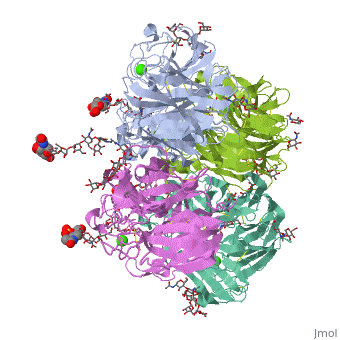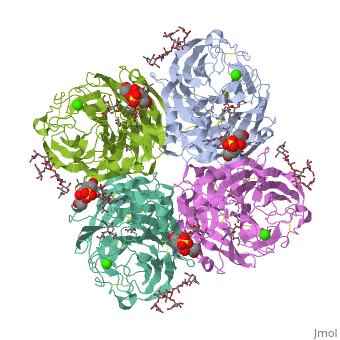2bat
THE STRUCTURE OF THE COMPLEX BETWEEN INFLUENZA VIRUS NEURAMINIDASE AND SIALIC ACID, THE VIRAL RECEPTOR
|
OverviewOverview
Crystallographic studies of neuraminidase-sialic acid complexes indicate, that sialic acid is distorted on binding the enzyme. Three arginine, residues on the enzyme interact with the carboxylate group of the sugar, which is observed to be equatorial to the saccharide ring as a consequence, of its distorted geometry. The glycosidic oxygen is positioned within, hydrogen-bonding distance of Asp-151, implicating this residue in, catalysis.
About this StructureAbout this Structure
2BAT is a Single protein structure of sequence from [1] with NAG, SIA and CA as ligands. Active as Exo-alpha-sialidase, with EC number 3.2.1.18 Full crystallographic information is available from OCA.
ReferenceReference
The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor., Varghese JN, McKimm-Breschkin JL, Caldwell JB, Kortt AA, Colman PM, Proteins. 1992 Nov;14(3):327-32. PMID:1438172
Page seeded by OCA on Wed Nov 21 08:41:02 2007