Ouabain
Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013.
Ouabain
|
|
Ouabain Structure and BindingOuabain Structure and Binding
Below is the structure of oubain in two dimensions. The molecule consists of a sugar bound to a modified cholesterol by a glycosidic linkage (hence glycoside). The hydroxyl groups surrounding much of the molecule, along with the esters at either end, contribute to its binding to the membrane bound sodium-potassium pump. can also be seen in three dimensions. With the molecular geometry and stereochemistry displayed in this way, one can see more clearly the distribution of polar carbon-oxygen and non-polar carbon-carbon bonds in the space surrounding the molecule. This makes visualizing the binding of the inhibitor much easier.
Cardiac Muscle and Ion Pump InhibitionCardiac Muscle and Ion Pump Inhibition
The inhibition of Na+/K+ ATPase has indirect effects on the contractile strength, or inotrophy, of cardiac muscle cells. Through the increase in cytoplasmic sodium concentration, a sodium-calcium exchanger is activated, thereby increasing the cellular concentration of calcium. Calcium is a second messenger in many signaling/regulatory pathways. Its release into the cytoplasm of muscle cells signals contraction, explaining the positive effect of low doses of ouabain on cardiac introphy. The image below depicts this effect of all cardiac glycosides on myocardial tissue. As is shown, the inhibition of the sodium-potassium pump causes elevated cytoplasmic sodium, which activates the sodium-calcium pump causing in increase in cytoplasmic calcium. This increases the force with which the cell contracts.
 If dosed correctly, ouabain proves to be effective in treating heart failure and arrhythmias. However, ouabain activity can indirectly contribute to the overloading of myocardial cells with sodium. Passive Na+ channels are usually inactivated when membrane potential is at its highest. When they re-open or fail to close, too much sodium can leak into the cell, causing the wasteful expenditure of ATP and/or uncoordinated muscle contraction. This is called late I-Na. With the rise of sodium and calcium, a Ca2+-calmodulin-dependent kinase is activated, in turn phosphorylating and activating passive Na+ channels. When the effects of ouabain are amplified in this way, the drug can cause more harm than good. Research has shown that an effective way to combat late I-Na is by the inhibition of the Ca2+-calmodulin-dependent kinase (Hoyer et al. 2011).
If dosed correctly, ouabain proves to be effective in treating heart failure and arrhythmias. However, ouabain activity can indirectly contribute to the overloading of myocardial cells with sodium. Passive Na+ channels are usually inactivated when membrane potential is at its highest. When they re-open or fail to close, too much sodium can leak into the cell, causing the wasteful expenditure of ATP and/or uncoordinated muscle contraction. This is called late I-Na. With the rise of sodium and calcium, a Ca2+-calmodulin-dependent kinase is activated, in turn phosphorylating and activating passive Na+ channels. When the effects of ouabain are amplified in this way, the drug can cause more harm than good. Research has shown that an effective way to combat late I-Na is by the inhibition of the Ca2+-calmodulin-dependent kinase (Hoyer et al. 2011).
SourcesSources
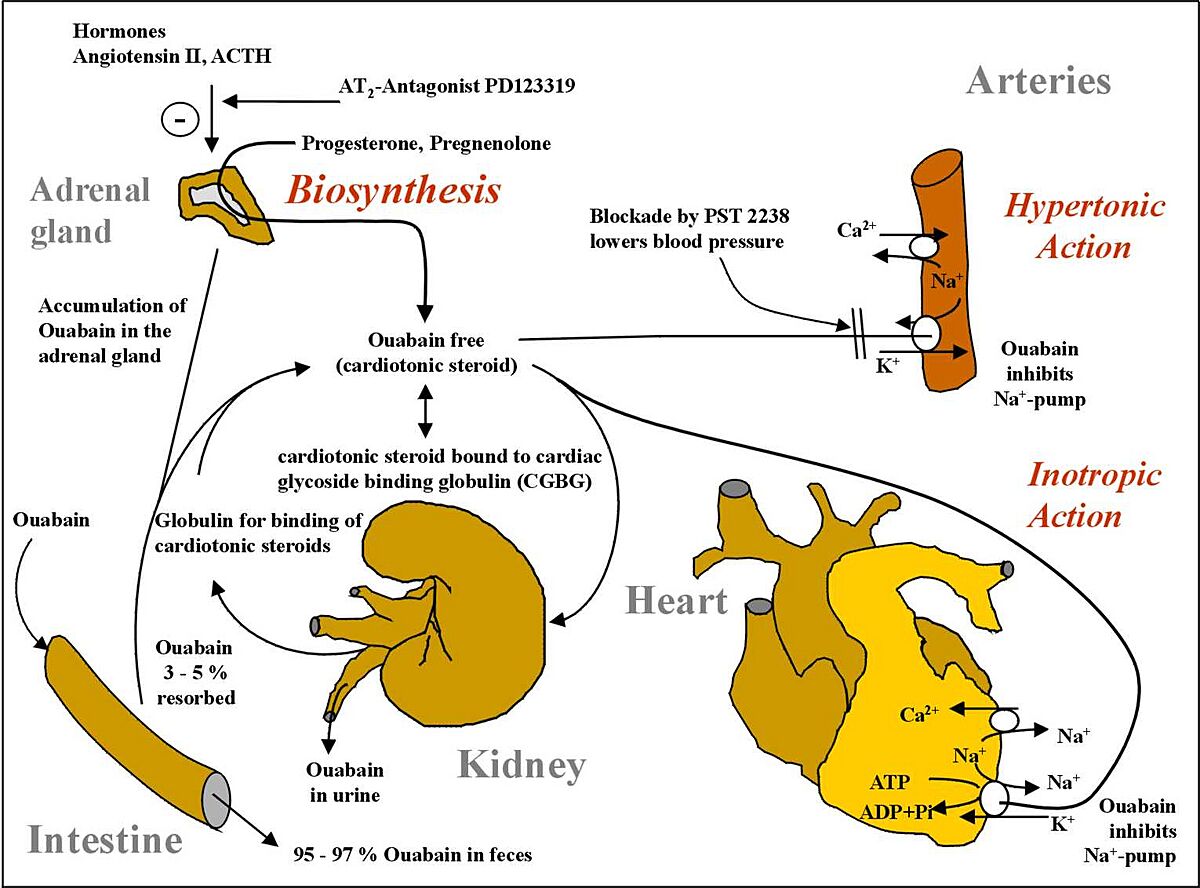
It was recently discovered that ouabain, long thought to be exclusively a plant product, is actually synthesized by animals, and secreted from the adrenal cortex to regulate body osmosis and cellular concentrations of sodium. The image below represents its biosynthesis and metabolism in humans.
Though there are currently synthetic schemes for the production of Ouabain, the compound is usually extracted from the plant sources Strophanthus gratus (left) and Acokanthera schimperi. Somalia is both the native habitat of these plants and the etymological origin of the name ouabain. Somalian tribes have historically used ouabain poisoned arrows for hunting. These arrows are capable of killing a hippopotamus, likely due to cardiac arrest.



