User:Liz Thomas/Sandbox 1
|
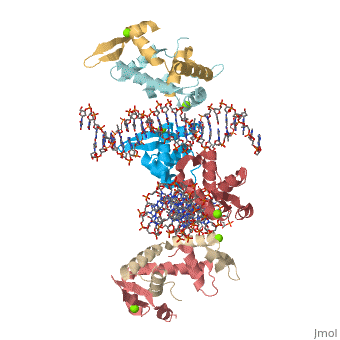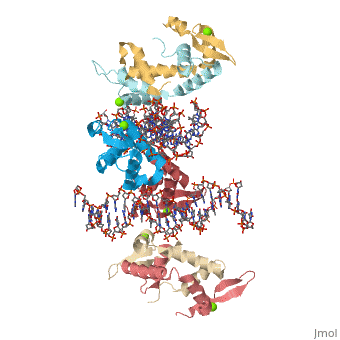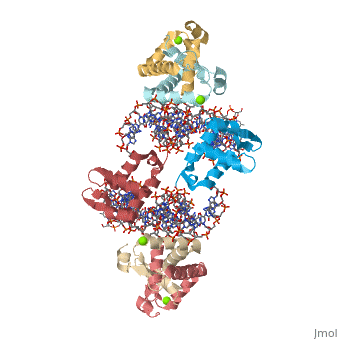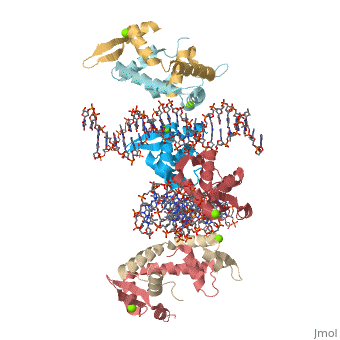
Crystal Structure of Foxp2 bound Specifically to DNA
New Article
|
| |||||||||
| 2a07, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | FOXP2, CAGH44, TNRC10 (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
IntroductionIntroduction
FOXP2 is a transcription factor containing a winged-helix DNA binding domain, and is necessary for proper development of the lungs and brain. It is located on human chromosome 7 (7q31), and comprises 715 amino acids in its major splice form. FOXP2 is a member of the FOX (forkhead box) family and FOXP (FOXP1-4) subfamily of proteins, all of which contain a 90 amino acid winged-helix DNA binding motif. The foxP subfamily contains motifs like a glutamine-rich region, a leucine zipper, a zinc finger, and a forkhead domain, and is notable for conducting domain swapping.
Evolutionary ConservationEvolutionary Conservation
Because FOXP2 is the first protein to be solidly linked to a human speech disorder, much investigation has focused on its evolutionary history in hopes of elucidating the molecular mechanism behind the uniqueness of human speech. Evidence suggests that the protein underwent a recent selective sweep within the same time frame as the purported rise of language in modern humans, supporting this idea. Interestingly, molecular data also shows neandertals and modern humans share the same sequence.
Chimpanzee, gorilla, and rhesus monkey FOXP2 sequences are identical. The human version differs from these primate versions by two amino acid changes: a threonine to asparagine at position 303 and asparagine to serine at position 325. Researchers have hypothesized that the human-specific change at 325 creates a potential phosphorylation site by protein kinase C, with a predicted corresponding change in the protein's transcriptional regulation.
DNA BindingDNA Binding
Domain SwappingDomain Swapping
Disease MutationsDisease Mutations
Disease mutations in FOXP2 and related proteins correspond to either the domain-swapping dimer interface or the DNA binding sequence. FOXP2 and FOXP3 are similar enough that the crystal structure of one can be used to explain the effects of mutation on structure in the other.
Arginine to Histidine at 553Arginine to Histidine at 553
This is the only mutation that has been characterized in the original FOXP2 protein.
Isoleucine to Valine at 363Isoleucine to Valine at 363
Alanine to Threonine at 385Alanine to Threonine at 385
Arginine to Tryptophan at 397Arginine to Tryptophan at 397
Phenylalanine to Cystine at 371 and Phenylalanine to Leucine at 371Phenylalanine to Cystine at 371 and Phenylalanine to Leucine at 371
ReferencesReferences
Stroud JC, Wu Y, Bates DL, Han A, Nowick K, Paabo S, Tong H, Chen L. 2006. Structure of the forkhead domain of FOXP2 bound to DNA. Structure 14: 159-166. doi:10.1016/j.str.2005.10.005
Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. 2001. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413: 519-523. doi:10.1038/35097076 PMID 11586359.
Krause J, Lalueza-Fox C, Orlando L, Enard W, Green RE, Burbano HA, Hublin JJ, Hanni C, Fortea J, de la Rasilla M, Bertrapetit J, Rosas A, Paabo S. 2007. The derived FOXP2 variant of modern humans was shared with neandertals. Current Biology 17:1-5. doi:10.1016/j.cub2007.10.2008
- ↑ Stroud JC, Wu Y, Bates DL, Han A, Nowick K, Paabo S, Tong H, Chen L. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006 Jan;14(1):159-66. PMID:16407075 doi:10.1016/j.str.2005.10.005