1aon
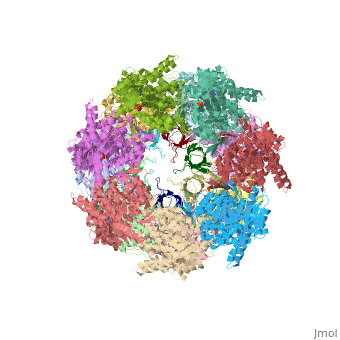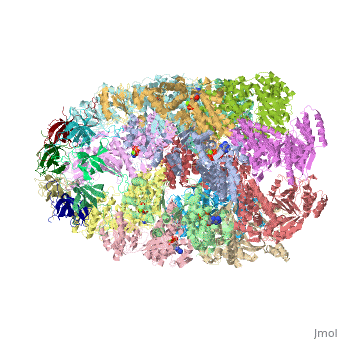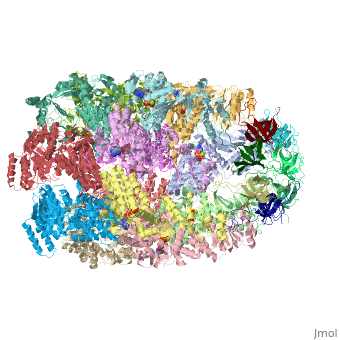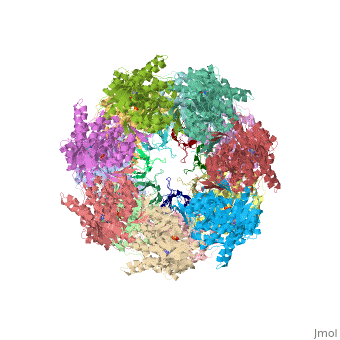
CRYSTAL STRUCTURE OF THE ASYMMETRIC CHAPERONIN COMPLEX GROEL/GROES/(ADP)7
|
OverviewOverview
Chaperonins assist protein folding with the consumption of ATP. They exist as multi-subunit protein assemblies comprising rings of subunits stacked back to back. In Escherichia coli, asymmetric intermediates of GroEL are formed with the co-chaperonin GroES and nucleotides bound only to one of the seven-subunit rings (the cis ring) and not to the opposing ring (the trans ring). The structure of the GroEL-GroES-(ADP)7 complex reveals how large en bloc movements of the cis ring's intermediate and apical domains enable bound GroES to stabilize a folding chamber with ADP confined to the cis ring. Elevation and twist of the apical domains double the volume of the central cavity and bury hydrophobic peptide-binding residues in the interface with GroES, as well as between GroEL subunits, leaving a hydrophilic cavity lining that is conducive to protein folding. An inward tilt of the cis equatorial domain causes an outward tilt in the trans ring that opposes the binding of a second GroES. When combined with new functional results, this negative allosteric mechanism suggests a model for an ATP-driven folding cycle that requires a double toroid.
About this StructureAbout this Structure
1AON is a Protein complex structure of sequences from Escherichia coli with and as ligands. The following page contains interesting information on the relation of 1AON with [Chaperones]. Full crystallographic information is available from OCA.
ReferenceReference
The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex., Xu Z, Horwich AL, Sigler PB, Nature. 1997 Aug 21;388(6644):741-50. PMID:9285585
Page seeded by OCA on Thu Feb 21 11:46:46 2008