Flagellar protein: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| Line 15: | Line 15: | ||
*'''FlhB''' is a component of the flagellar secretion system and has a role in protein export<ref>PMID: 23874605</ref>. | *'''FlhB''' is a component of the flagellar secretion system and has a role in protein export<ref>PMID: 23874605</ref>. | ||
*'''FlhE''' acts as chaperone to regulate flow of proteins through the flagellar type III secretion system<ref>PMID: 22435757</ref>. | *'''FlhE''' acts as chaperone to regulate flow of proteins through the flagellar type III secretion system<ref>PMID: 22435757</ref>. | ||
*'''FllF''' is a signal recognition particle-like GTPase regulating flagellar number and polarity<ref>PMID: 32039533</ref>. | |||
*'''FliD''' regulates filament assembly by chaperoning [[Flagellin]] proteins<ref>PMID: 27664419</ref>. | *'''FliD''' regulates filament assembly by chaperoning [[Flagellin]] proteins<ref>PMID: 27664419</ref>. | ||
For further information, please see [http://en.wikipedia.org/wiki/Flagellum Flagellum at Wikipedia]. | For further information, please see [http://en.wikipedia.org/wiki/Flagellum Flagellum at Wikipedia]. | ||
Revision as of 12:12, 24 June 2024
Function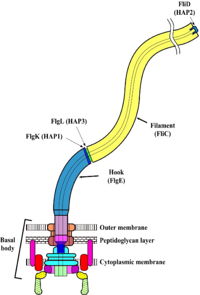
Flagella (singular: flagellum) enable bacteria to swim towards sources of nutrition, and away from sources of toxins. Such directed motility is termed chemotaxis. Rapid swimming helps Bdellovibrio penetrate and parasitize their host bacteria, but flagella are not always essential for virulence[1]. Flagella are important in responses to quorum sensing[2] and biofilm formation[3][4]. Flagella may also be involved in functions other than motility[5].
For further information, please see Flagellum at Wikipedia. The bacterial flagellum is made up of about 25 different proteins. There are only a few copies of some proteins, and tens of thousands of copies of the filament protein, FliC. The flagellum is made up of three major regions, as follows. MotorAt the base of a bacterial flagellum is a reversible motor, also called the basal body. The source of energy driving the motor is an electromotive gradient of, in some bacteria, protons (hydrogen ions, H+) or, in other bacteria, sodium ions (Na+). The gradient has a higher concentration of ions outside the cell, and a lower concentration of ions inside the cell. Ions flow from outside to inside the bacterial cell, passing through the motor and driving its rotation by a mechanism which is poorly understood. For more details see Flagellar biosynthetic protein Filament (Propeller)The flagellar filament is a relatively rigid, helical rod, typically many times the length of the bacterial cell. Many motile bacteria, including Salmonella, have multiple flagella extending from each cell. Rotation of the filaments by the motor is what propels the cell. More... For more details see Flagellar proteins and Flagellar filament of bacteria. Hook (Universal Joint)The filament is attached to the motor with the flagellar hook, which is a molecular universal joint. The hook is flexible, allowing the angle between the filament and the bacterial cell surface to change over a wide range. However, the hook efficiently transmits torque from the motor to the filament, causing it to rotate. For details see Assembly
During assembly of the flagellum, its protein components are transported through hollow cores of the basal body, hook and filament, assembling at the end of the nascent flagellum[17]. The Namba Group has prepared a movie illustrating their understanding of the assembly process as of about 2004. 3D structures of flagellar proteinFlagellar protein 3D structures
|
| |||||||||||||
Lists of Flagellar StructuresLists of Flagellar Structures
These are automatically-generated lists of PDB codes.
- Special:Prefixindex/Category:Flagell includes categories beginning with Flagellar, Flagellin, Flagella, Flagellum.
- Special:Prefixindex/Category:Bacterial flagell
- Category:Chlamydomonas flagella
- Category:The bacterial flagellar motor
- Category:Putative flagellar motor switch protein flin
and there are undoubtedly other flagellum-related Categories ...
See AlsoSee Also
Within Proteopedia:
External Links
- Flagellum at Wikipedia
- Protonic Nanomachine Group, Osaka University
- MOVIES from the Protonic Nanomachine Project, Osaka University
</StructureSection>
References and NotesReferences and Notes
- ↑ Lockman HA, Curtiss R 3rd. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990 Jan;58(1):137-43. PMID:2152887
- ↑ Daniels R, Vanderleyden J, Michiels J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev. 2004 Jun;28(3):261-89. PMID:15449604
- ↑ Yildiz FH, Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009 Mar;17(3):109-18. Epub 2009 Feb 21. PMID:19231189 doi:10.1016/j.tim.2008.12.004
- ↑ Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J Bacteriol. 2007 Jun;189(12):4418-24. Epub 2007 Apr 6. PMID:17416647 doi:10.1128/JB.01967-06
- ↑ Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007 Oct;15(10):456-61. Epub 2007 Oct 24. PMID:17920274 doi:10.1016/j.tim.2007.09.006
- ↑ Matsunami H, Yoon YH, Meshcheryakov VA, Namba K, Samatey FA. Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica. Sci Rep. 2016 Jun 7;6:27399. doi: 10.1038/srep27399. PMID:27273476 doi:http://dx.doi.org/10.1038/srep27399
- ↑ Ohnishi K, Ohto Y, Aizawa S, Macnab RM, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994 Apr;176(8):2272-81. PMID:8157595
- ↑ You Y, Ye F, Mao W, Yang H, Lai J, Deng S. An overview of the structure and function of the flagellar hook FlgE protein. World J Microbiol Biotechnol. 2023 Mar 21;39(5):126. PMID:36941455 doi:10.1007/s11274-023-03568-6
- ↑ Saijo-Hamano Y, Uchida N, Namba K, Oosawa K. In vitro characterization of FlgB, FlgC, FlgF, FlgG, and FliE, flagellar basal body proteins of Salmonella. J Mol Biol. 2004 May 28;339(2):423-35. PMID:15136044 doi:10.1016/j.jmb.2004.03.070
- ↑ Herlihey FA, Moynihan PJ, Clarke AJ. The essential protein for bacterial flagella formation FlgJ functions as a β-N-acetylglucosaminidase. J Biol Chem. 2014 Nov 7;289(45):31029-42. PMID:25248745 doi:10.1074/jbc.M114.603944
- ↑ Bulieris PV, Shaikh NH, Freddolino PL, Samatey FA. Structure of FlgK reveals the divergence of the bacterial Hook-Filament Junction of Campylobacter. Sci Rep. 2017 Nov 16;7(1):15743. doi: 10.1038/s41598-017-15837-0. PMID:29147015 doi:http://dx.doi.org/10.1038/s41598-017-15837-0
- ↑ Inoue Y, Kinoshita M, Kida M, Takekawa N, Namba K, Imada K, Minamino T. The FlhA linker mediates flagellar protein export switching during flagellar assembly. Commun Biol. 2021 May 31;4(1):646. PMID:34059784 doi:10.1038/s42003-021-02177-z
- ↑ Meshcheryakov VA, Barker CS, Kostyukova AS, Samatey FA. Function of FlhB, a membrane protein implicated in the bacterial flagellar type III secretion system. PLoS One. 2013 Jul 11;8(7):e68384. doi: 10.1371/journal.pone.0068384. Print 2013. PMID:23874605 doi:http://dx.doi.org/10.1371/journal.pone.0068384
- ↑ Lee J, Harshey RM. Loss of FlhE in the flagellar Type III secretion system allows proton influx into Salmonella and Escherichia coli. Mol Microbiol. 2012 May;84(3):550-65. PMID:22435757 doi:10.1111/j.1365-2958.2012.08043.x
- ↑ Zhang K, He J, Cantalano C, Guo Y, Liu J, Li C. FlhF regulates the number and configuration of periplasmic flagella in Borrelia burgdorferi. Mol Microbiol. 2020 Jun;113(6):1122-1139. PMID:32039533 doi:10.1111/mmi.14482
- ↑ Postel S, Deredge D, Bonsor DA, Yu X, Diederichs K, Helmsing S, Vromen A, Friedler A, Hust M, Egelman EH, Beckett D, Wintrode PL, Sundberg EJ. Bacterial flagellar capping proteins adopt diverse oligomeric states. Elife. 2016 Sep 24;5. pii: e18857. doi: 10.7554/eLife.18857. PMID:27664419 doi:http://dx.doi.org/10.7554/eLife.18857
- ↑ Minamino T, Imada K, Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst. 2008 Nov;4(11):1105-15. Epub 2008 Sep 24. PMID:18931786 doi:10.1039/b808065h