1tha: Difference between revisions
New page: left|200px<br /> <applet load="1tha" size="450" color="white" frame="true" align="right" spinBox="true" caption="1tha, resolution 2.0Å" /> '''MECHANISM OF MOLECUL... |
No edit summary |
||
| Line 6: | Line 6: | ||
==Overview== | ==Overview== | ||
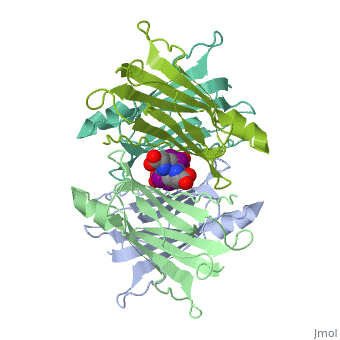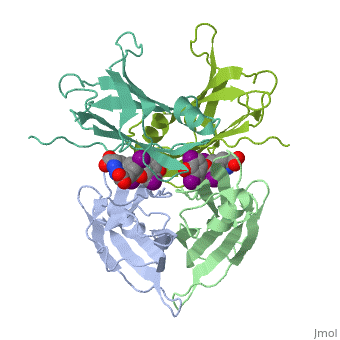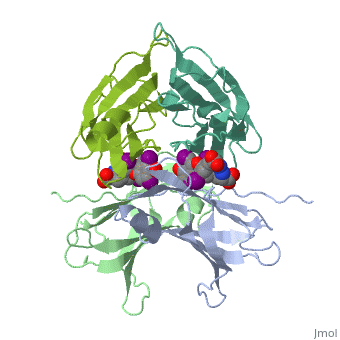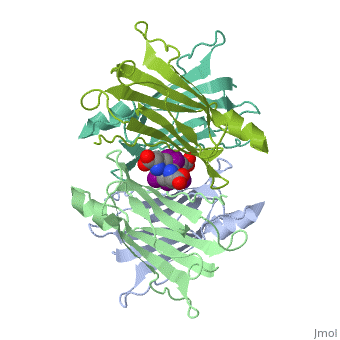
The three-dimensional structure of the thyroid hormone metabolite, 3,3'-diiodo-L-thyronine (3,3'-T2), complex with human serum transthyretin, (TTR) has been refined to R = 18.5% for 8-2 A resolution data. This is the, first detailed description of a thyroid hormone metabolite binding to a, thyroid transport protein. The four TTR monomeric subunits form a tetramer, in the same manner as the native transthyretin reported earlier (Blake, C., C. F., Geisow, M. J., Oatley, S. J., Rerat, B., and Rerat, C. (1978) J., Mol. Biol. 121, 339-356). The two hormone binding sites of the TTR, tetramer are occupied by 3,3'-T2. A statistical disorder model for the, ligand was applied with a 50% occupancy to account for the discrepancy, between the crystallographic 2-fold symmetry of the binding sites and the, lack of such symmetry for 3,3'-T2. The bound metabolite has an overall, transoid conformation with the either bridge intermediate between skewed, and perpendicular. The hormone metabolite is bound 3.5 A deeper and with a, different orientation in the channel than observed for thyroxine (T4), thereby revealing the presence of another set of halogen binding sites, close to the center of the tetramer. When compared with the binding of T4, these data show that the 3-iodine of 3,3'-T2 occupies the same site as the, 3'-iodine of T4, and the metabolite 3'-iodine occupies the water site, observed in the T4 complex. The binding affinity of 3,3'-T2, which is, 100-fold lower than that of T4, reflects the lack of the second pair of, iodine atoms interacting in the channel. In order to understand the, tighter binding of T4 observed in the Ala-109----Thr mutant, modeling, studies were carried out that indicate that this modification could, shorten the contacts between thyroid hormone iodines and residues 108-110, of the binding site. | The three-dimensional structure of the thyroid hormone metabolite, 3,3'-diiodo-L-thyronine (3,3'-T2), complex with human serum transthyretin, (TTR) has been refined to R = 18.5% for 8-2 A resolution data. This is the, first detailed description of a thyroid hormone metabolite binding to a, thyroid transport protein. The four TTR monomeric subunits form a tetramer, in the same manner as the native transthyretin reported earlier (Blake, C., C. F., Geisow, M. J., Oatley, S. J., Rerat, B., and Rerat, C. (1978) J., Mol. Biol. 121, 339-356). The two hormone binding sites of the TTR, tetramer are occupied by 3,3'-T2. A statistical disorder model for the, ligand was applied with a 50% occupancy to account for the discrepancy, between the crystallographic 2-fold symmetry of the binding sites and the, lack of such symmetry for 3,3'-T2. The bound metabolite has an overall, transoid conformation with the either bridge intermediate between skewed, and perpendicular. The hormone metabolite is bound 3.5 A deeper and with a, different orientation in the channel than observed for thyroxine (T4), thereby revealing the presence of another set of halogen binding sites, close to the center of the tetramer. When compared with the binding of T4, these data show that the 3-iodine of 3,3'-T2 occupies the same site as the, 3'-iodine of T4, and the metabolite 3'-iodine occupies the water site, observed in the T4 complex. The binding affinity of 3,3'-T2, which is, 100-fold lower than that of T4, reflects the lack of the second pair of, iodine atoms interacting in the channel. In order to understand the, tighter binding of T4 observed in the Ala-109----Thr mutant, modeling, studies were carried out that indicate that this modification could, shorten the contacts between thyroid hormone iodines and residues 108-110, of the binding site. | ||
==Disease== | |||
Known diseases associated with this structure: Amyloid neuropathy, familial, several allelic types OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=176300 176300]], Amyloidosis, senile systemic OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=176300 176300]], Carpal tunnel syndrome, familial OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=176300 176300]], Dystransthyretinemic hyperthyroxinemia OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=176300 176300]] | |||
==About this Structure== | ==About this Structure== | ||
| Line 21: | Line 24: | ||
[[Category: transport(thyroxine)]] | [[Category: transport(thyroxine)]] | ||
''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on Mon Nov 12 19:25:02 2007'' | ||
Revision as of 20:18, 12 November 2007
|
MECHANISM OF MOLECULAR RECOGNITION. STRUCTURAL ASPECTS OF 3,3'-DIIODO-L-THYRONINE BINDING TO HUMAN SERUM TRANSTHYRETIN
OverviewOverview
The three-dimensional structure of the thyroid hormone metabolite, 3,3'-diiodo-L-thyronine (3,3'-T2), complex with human serum transthyretin, (TTR) has been refined to R = 18.5% for 8-2 A resolution data. This is the, first detailed description of a thyroid hormone metabolite binding to a, thyroid transport protein. The four TTR monomeric subunits form a tetramer, in the same manner as the native transthyretin reported earlier (Blake, C., C. F., Geisow, M. J., Oatley, S. J., Rerat, B., and Rerat, C. (1978) J., Mol. Biol. 121, 339-356). The two hormone binding sites of the TTR, tetramer are occupied by 3,3'-T2. A statistical disorder model for the, ligand was applied with a 50% occupancy to account for the discrepancy, between the crystallographic 2-fold symmetry of the binding sites and the, lack of such symmetry for 3,3'-T2. The bound metabolite has an overall, transoid conformation with the either bridge intermediate between skewed, and perpendicular. The hormone metabolite is bound 3.5 A deeper and with a, different orientation in the channel than observed for thyroxine (T4), thereby revealing the presence of another set of halogen binding sites, close to the center of the tetramer. When compared with the binding of T4, these data show that the 3-iodine of 3,3'-T2 occupies the same site as the, 3'-iodine of T4, and the metabolite 3'-iodine occupies the water site, observed in the T4 complex. The binding affinity of 3,3'-T2, which is, 100-fold lower than that of T4, reflects the lack of the second pair of, iodine atoms interacting in the channel. In order to understand the, tighter binding of T4 observed in the Ala-109----Thr mutant, modeling, studies were carried out that indicate that this modification could, shorten the contacts between thyroid hormone iodines and residues 108-110, of the binding site.
DiseaseDisease
Known diseases associated with this structure: Amyloid neuropathy, familial, several allelic types OMIM:[176300], Amyloidosis, senile systemic OMIM:[176300], Carpal tunnel syndrome, familial OMIM:[176300], Dystransthyretinemic hyperthyroxinemia OMIM:[176300]
About this StructureAbout this Structure
1THA is a Single protein structure of sequence from Homo sapiens with T33 as ligand. The following page contains interesting information on the relation of 1THA with [Serum Albumin]. Full crystallographic information is available from OCA.
ReferenceReference
Mechanism of molecular recognition. Structural aspects of 3,3'-diiodo-L-thyronine binding to human serum transthyretin., Wojtczak A, Luft J, Cody V, J Biol Chem. 1992 Jan 5;267(1):353-7. PMID:1730601
Page seeded by OCA on Mon Nov 12 19:25:02 2007