3ryf: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
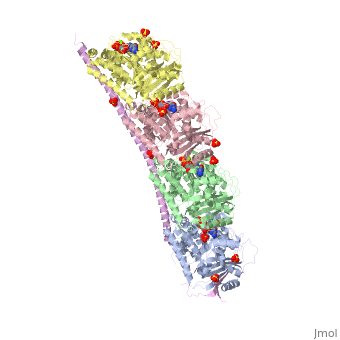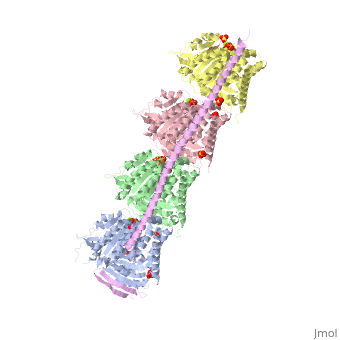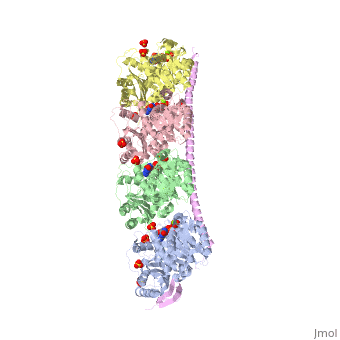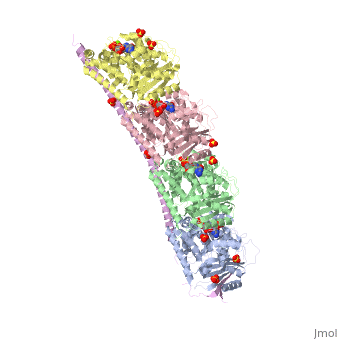
<StructureSection load='3ryf' size='340' side='right'caption='[[3ryf]], [[Resolution|resolution]] 2.52Å' scene=''> | <StructureSection load='3ryf' size='340' side='right'caption='[[3ryf]], [[Resolution|resolution]] 2.52Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[3ryf]] is a 5 chain structure with sequence from [https://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[3ryf]] is a 5 chain structure with sequence from [https://en.wikipedia.org/wiki/Ovis_aries Ovis aries] and [https://en.wikipedia.org/wiki/Rattus_norvegicus Rattus norvegicus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3RYF OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3RYF FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.52Å</td></tr> | ||
<tr id=' | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ACE:ACETYL+GROUP'>ACE</scene>, <scene name='pdbligand=GTP:GUANOSINE-5-TRIPHOSPHATE'>GTP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3ryf FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3ryf OCA], [https://pdbe.org/3ryf PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3ryf RCSB], [https://www.ebi.ac.uk/pdbsum/3ryf PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3ryf ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3ryf FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3ryf OCA], [https://pdbe.org/3ryf PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3ryf RCSB], [https://www.ebi.ac.uk/pdbsum/3ryf PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3ryf ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
[https://www.uniprot.org/uniprot/D0VWZ0_SHEEP D0VWZ0_SHEEP] Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505][SAAS:SAAS023123_004_019801] | |||
==See Also== | ==See Also== | ||
| Line 26: | Line 15: | ||
*[[Stathmin-4 3D structures|Stathmin-4 3D structures]] | *[[Stathmin-4 3D structures|Stathmin-4 3D structures]] | ||
*[[Tubulin 3D Structures|Tubulin 3D Structures]] | *[[Tubulin 3D Structures|Tubulin 3D Structures]] | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: Ovis aries]] | [[Category: Ovis aries]] | ||
[[Category: | [[Category: Rattus norvegicus]] | ||
[[Category: | [[Category: Gigant B]] | ||
[[Category: | [[Category: Knossow M]] | ||
[[Category: | [[Category: Nawrotek A]] | ||
Revision as of 15:40, 14 March 2024
GTP-Tubulin: RB3 Stathmin-like domain complexGTP-Tubulin: RB3 Stathmin-like domain complex
Structural highlights
FunctionD0VWZ0_SHEEP Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505][SAAS:SAAS023123_004_019801] See Also |
| ||||||||||||||||||