2xg7: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='2xg7' size='340' side='right'caption='[[2xg7]], [[Resolution|resolution]] 3.45Å' scene=''> | <StructureSection load='2xg7' size='340' side='right'caption='[[2xg7]], [[Resolution|resolution]] 3.45Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2xg7]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[2xg7]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2XG7 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2XG7 FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.45Å</td></tr> | ||
<tr id=' | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2xg7 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2xg7 OCA], [https://pdbe.org/2xg7 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2xg7 RCSB], [https://www.ebi.ac.uk/pdbsum/2xg7 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2xg7 ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2xg7 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2xg7 OCA], [https://pdbe.org/2xg7 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2xg7 RCSB], [https://www.ebi.ac.uk/pdbsum/2xg7 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2xg7 ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
[https://www.uniprot.org/uniprot/BST2_HUMAN BST2_HUMAN] IFN-induced antiviral host restriction factor which efficiently blocks the release of diverse mammalian enveloped viruses by directly tethering nascent virions to the membranes of infected cells. Acts as a direct physical tether, holding virions to the cell membrane and linking virions to each other. The tethered virions can be internalized by endocytosis and subsequently degraded or they can remain on the cell surface. In either case, their spread as cell-free virions is restricted. Its target viruses belong to diverse families, including retroviridae: human immunodeficiency virus type 1 (HIV-1), human immunodeficiency virus type 2 (HIV-2), simian immunodeficiency viruses (SIVs), equine infectious anemia virus (EIAV), feline immunodeficiency virus (FIV), prototype foamy virus (PFV), Mason-Pfizer monkey virus (MPMV), human T-cell leukemia virus type 1 (HTLV-1), Rous sarcoma virus (RSV) and murine leukemia virus (MLV), flavivirideae: hepatitis C virus (HCV), filoviridae: ebola virus (EBOV) and marburg virus (MARV), arenaviridae: lassa virus (LASV) and machupo virus (MACV), herpesviridae: kaposis sarcoma-associated herpesvirus (KSHV), rhabdoviridae: vesicular stomatitis virus (VSV), orthomyxoviridae: influenza A virus, and paramyxoviridae: nipah virus. Can inhibit cell surface proteolytic activity of MMP14 causing decreased activation of MMP15 which results in inhibition of cell growth and migration. Can stimulate signaling by LILRA4/ILT7 and consequently provide negative feedback to the production of IFN by plasmacytoid dendritic cells in response to viral infection. Plays a role in the organization of the subapical actin cytoskeleton in polarized epithelial cells.<ref>PMID:18342597</ref> <ref>PMID:18200009</ref> <ref>PMID:19879838</ref> <ref>PMID:19564354</ref> <ref>PMID:19036818</ref> <ref>PMID:19179289</ref> <ref>PMID:20686043</ref> <ref>PMID:20943977</ref> <ref>PMID:20419159</ref> <ref>PMID:21529378</ref> <ref>PMID:21621240</ref> <ref>PMID:22065321</ref> <ref>PMID:22520941</ref> <ref>PMID:20399176</ref> <ref>PMID:20940320</ref> | |||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
| Line 26: | Line 26: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Homo sapiens]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: Steiner | [[Category: Steiner RA]] | ||
Latest revision as of 13:30, 20 December 2023
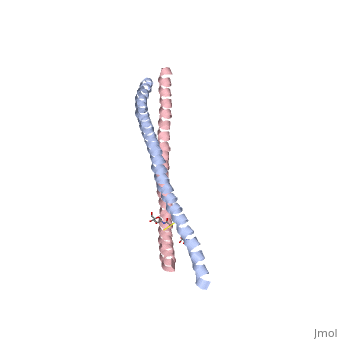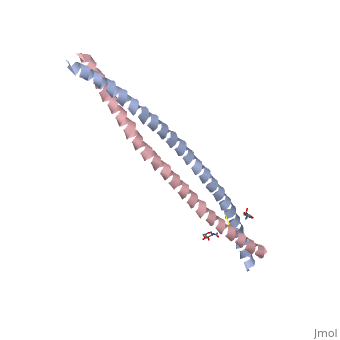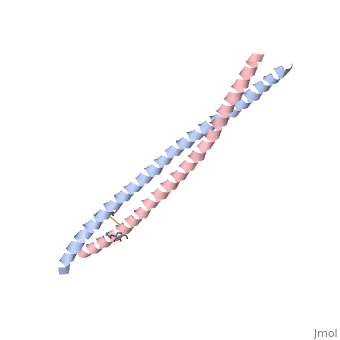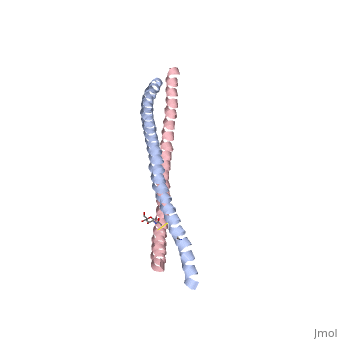
Crystal Structure of BST2-Tetherin Ectodomain expressed in HEK293T cellsCrystal Structure of BST2-Tetherin Ectodomain expressed in HEK293T cells
Structural highlights
FunctionBST2_HUMAN IFN-induced antiviral host restriction factor which efficiently blocks the release of diverse mammalian enveloped viruses by directly tethering nascent virions to the membranes of infected cells. Acts as a direct physical tether, holding virions to the cell membrane and linking virions to each other. The tethered virions can be internalized by endocytosis and subsequently degraded or they can remain on the cell surface. In either case, their spread as cell-free virions is restricted. Its target viruses belong to diverse families, including retroviridae: human immunodeficiency virus type 1 (HIV-1), human immunodeficiency virus type 2 (HIV-2), simian immunodeficiency viruses (SIVs), equine infectious anemia virus (EIAV), feline immunodeficiency virus (FIV), prototype foamy virus (PFV), Mason-Pfizer monkey virus (MPMV), human T-cell leukemia virus type 1 (HTLV-1), Rous sarcoma virus (RSV) and murine leukemia virus (MLV), flavivirideae: hepatitis C virus (HCV), filoviridae: ebola virus (EBOV) and marburg virus (MARV), arenaviridae: lassa virus (LASV) and machupo virus (MACV), herpesviridae: kaposis sarcoma-associated herpesvirus (KSHV), rhabdoviridae: vesicular stomatitis virus (VSV), orthomyxoviridae: influenza A virus, and paramyxoviridae: nipah virus. Can inhibit cell surface proteolytic activity of MMP14 causing decreased activation of MMP15 which results in inhibition of cell growth and migration. Can stimulate signaling by LILRA4/ILT7 and consequently provide negative feedback to the production of IFN by plasmacytoid dendritic cells in response to viral infection. Plays a role in the organization of the subapical actin cytoskeleton in polarized epithelial cells.[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] Publication Abstract from PubMedHIV-1 and other enveloped viruses can be restricted by a host cellular protein called BST2/tetherin that prevents release of budded viruses from the cell surface. Mature BST2 contains a small cytosolic region, a predicted transmembrane helix, and an extracellular domain with a C-terminal GPI anchor. To advance understanding of BST2 function, we have determined a 2.6 A crystal structure of the extracellular domain of the bacterially expressed recombinant human protein, residues 47-152, under reducing conditions. The structure forms a single long helix that associates as a parallel dimeric coiled coil over its C-terminal two-thirds, while the N-terminal third forms an antiparallel four-helix bundle with another dimer, creating a global tetramer. We also report the 3.45 A resolution structure of BST2(51-151) prepared by expression as a secreted protein in HEK293T cells. This oxidized construct forms a dimer in the crystal that is superimposable with the reduced protein over the C-terminal two-thirds of the molecule, and its N terminus suggests pronounced flexibility. Hydrodynamic data demonstrated that BST2 formed a stable tetramer under reducing conditions and a dimer when oxidized to form disulfide bonds. A mutation that selectively disrupted the tetramer (L70D) increased protein expression modestly but only reduced antiviral activity by approximately threefold. Our data raise the possibility that BST2 may function as a tetramer at some stage, such as during trafficking, and strongly support a model in which the primary functional state of BST2 is a parallel disulfide-bound coiled coil that displays flexibility toward its N terminus. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations.,Schubert HL, Zhai Q, Sandrin V, Eckert DM, Garcia-Maya M, Saul L, Sundquist WI, Steiner RA, Hill CP Proc Natl Acad Sci U S A. 2010 Sep 29. PMID:20880831[16] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||