1w2t: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='1w2t' size='340' side='right'caption='[[1w2t]], [[Resolution|resolution]] 1.87Å' scene=''> | <StructureSection load='1w2t' size='340' side='right'caption='[[1w2t]], [[Resolution|resolution]] 1.87Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1w2t]] is a 6 chain structure with sequence from [https://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[1w2t]] is a 6 chain structure with sequence from [https://en.wikipedia.org/wiki/Thermotoga_maritima_MSB8 Thermotoga maritima MSB8]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1W2T OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1W2T FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.87Å</td></tr> | ||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CIT:CITRIC+ACID'>CIT</scene>, <scene name='pdbligand=FRU:FRUCTOSE'>FRU</scene>, <scene name='pdbligand=GLA:ALPHA+D-GALACTOSE'>GLA</scene>, <scene name='pdbligand=GLC:ALPHA-D-GLUCOSE'>GLC</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1w2t FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1w2t OCA], [https://pdbe.org/1w2t PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1w2t RCSB], [https://www.ebi.ac.uk/pdbsum/1w2t PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1w2t ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1w2t FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1w2t OCA], [https://pdbe.org/1w2t PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1w2t RCSB], [https://www.ebi.ac.uk/pdbsum/1w2t PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1w2t ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | |||
[https://www.uniprot.org/uniprot/BFRA_THEMA BFRA_THEMA] Hydrolysis of sucrose, raffinose, inulin and levan. Specific for the fructose moiety and the beta-anomeric configuration of the glycosidic linkages of its substrates. The enzyme released fructose from sucrose and raffinose, and the fructose polymer inulin is hydrolyzed quantitatively in an exo-type fashion. | |||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 35: | Line 36: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: | [[Category: Thermotoga maritima MSB8]] | ||
[[Category: Alberto | [[Category: Alberto F]] | ||
[[Category: Czjzek | [[Category: Czjzek M]] | ||
[[Category: Henrissat | [[Category: Henrissat B]] | ||
Latest revision as of 16:13, 13 December 2023
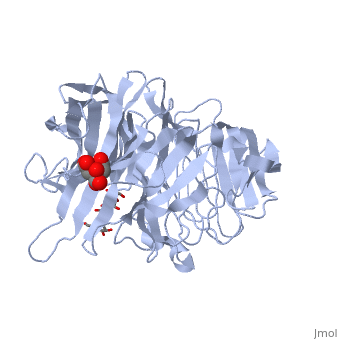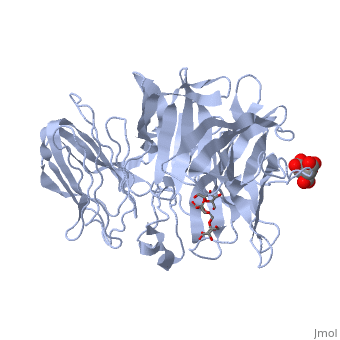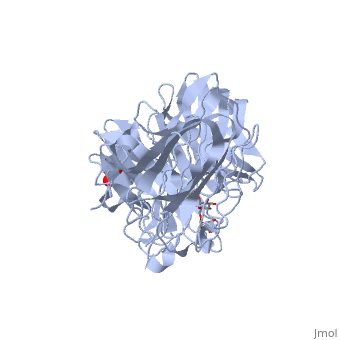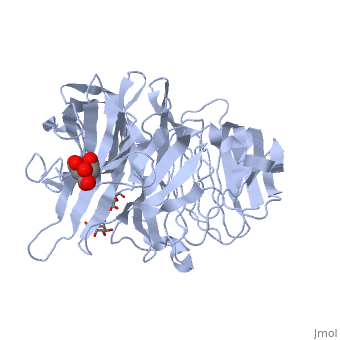
beta-fructosidase from Thermotoga maritima in complex with raffinosebeta-fructosidase from Thermotoga maritima in complex with raffinose
Structural highlights
FunctionBFRA_THEMA Hydrolysis of sucrose, raffinose, inulin and levan. Specific for the fructose moiety and the beta-anomeric configuration of the glycosidic linkages of its substrates. The enzyme released fructose from sucrose and raffinose, and the fructose polymer inulin is hydrolyzed quantitatively in an exo-type fashion. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThermotoga maritima invertase (beta-fructosidase), a member of the glycoside hydrolase family GH-32, readily releases beta-D-fructose from sucrose, raffinose and fructan polymers such as inulin. These carbohydrates represent major carbon and energy sources for prokaryotes and eukaryotes. The invertase cleaves beta-fructopyranosidic linkages by a double-displacement mechanism, which involves a nucleophilic aspartate and a catalytic glutamic acid acting as a general acid/base. The three-dimensional structure of invertase shows a bimodular enzyme with a five bladed beta-propeller catalytic domain linked to a beta-sandwich of unknown function. In the present study we report the crystal structure of the inactivated invertase in interaction with the natural substrate molecule alpha-D-galactopyranosyl-(1,6)-alpha-D-glucopyranosyl-beta-D-fructofuranos ide (raffinose) at 1.87 A (1 A=0.1 nm) resolution. The structural analysis of the complex reveals the presence of three binding-subsites, which explains why T. maritima invertase exhibits a higher affinity for raffinose than sucrose, but a lower catalytic efficiency with raffinose as substrate than with sucrose. Crystal structure of inactivated Thermotoga maritima invertase in complex with the trisaccharide substrate raffinose.,Alberto F, Jordi E, Henrissat B, Czjzek M Biochem J. 2006 May 1;395(3):457-62. PMID:16411890[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||