1hyb: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
<StructureSection load='1hyb' size='340' side='right'caption='[[1hyb]], [[Resolution|resolution]] 2.00Å' scene=''> | <StructureSection load='1hyb' size='340' side='right'caption='[[1hyb]], [[Resolution|resolution]] 2.00Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1hyb]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[1hyb]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Methanothermobacter_thermautotrophicus Methanothermobacter thermautotrophicus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1HYB OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1HYB FirstGlance]. <br> | ||
</td></tr><tr id=' | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2Å</td></tr> | ||
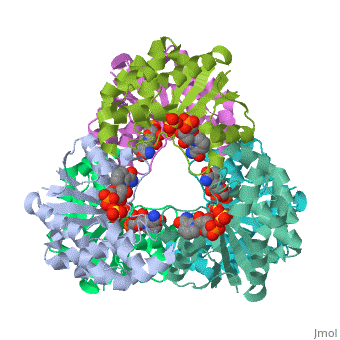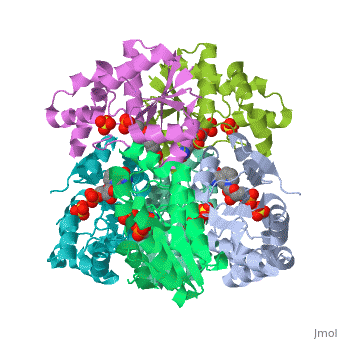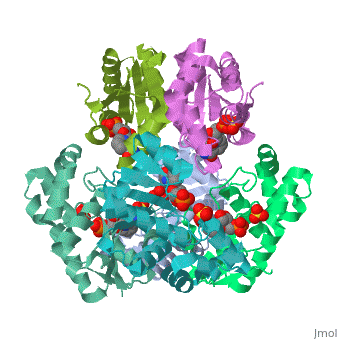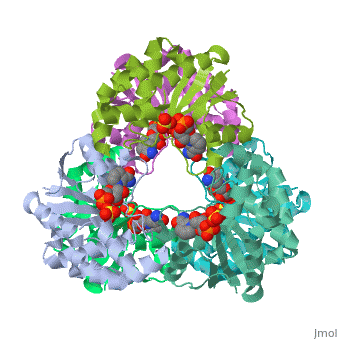
<tr id=' | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NMN:BETA-NICOTINAMIDE+RIBOSE+MONOPHOSPHATE'>NMN</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1hyb FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1hyb OCA], [https://pdbe.org/1hyb PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1hyb RCSB], [https://www.ebi.ac.uk/pdbsum/1hyb PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1hyb ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1hyb FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1hyb OCA], [https://pdbe.org/1hyb PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1hyb RCSB], [https://www.ebi.ac.uk/pdbsum/1hyb PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1hyb ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | |||
[https://www.uniprot.org/uniprot/NADM_METTH NADM_METTH] | |||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 36: | Line 37: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
[[Category: | [[Category: Methanothermobacter thermautotrophicus]] | ||
[[Category: Christendat | [[Category: Christendat D]] | ||
[[Category: Edwards | [[Category: Edwards AM]] | ||
[[Category: Kimber | [[Category: Kimber MS]] | ||
[[Category: Pai | [[Category: Pai EF]] | ||
[[Category: Saridakis | [[Category: Saridakis V]] | ||
Latest revision as of 09:18, 9 August 2023
CRYSTAL STRUCTURE OF AN ACTIVE SITE MUTANT OF METHANOBACTERIUM THERMOAUTOTROPHICUM NICOTINAMIDE MONONUCLEOTIDE ADENYLYLTRANSFERASECRYSTAL STRUCTURE OF AN ACTIVE SITE MUTANT OF METHANOBACTERIUM THERMOAUTOTROPHICUM NICOTINAMIDE MONONUCLEOTIDE ADENYLYLTRANSFERASE
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedNicotinamide mononucleotide adenylyltransferase (NMNATase) catalyzes the linking of NMN(+) or NaMN(+) with ATP, which in all organisms is one of the common step in the synthesis of the ubiquitous coenzyme NAD(+), via both de novo and salvage biosynthetic pathways. The structure of Methanobacterium thermoautotrophicum NMNATase determined using multiwavelength anomalous dispersion phasing revealed a nucleotide-binding fold common to nucleotidyltransferase proteins. An NAD(+) molecule and a sulfate ion were bound in the active site allowing the identification of residues involved in product binding. In addition, the role of the conserved (16)HXGH(19) active site motif in catalysis was probed by mutagenic, enzymatic and crystallographic techniques, including the characterization of an NMN(+)/SO4(2-) complex of mutant H19A NMNATase. Insights into ligand binding and catalysis of a central step in NAD+ synthesis: structures of Methanobacterium thermoautotrophicum NMN adenylyltransferase complexes.,Saridakis V, Christendat D, Kimber MS, Dharamsi A, Edwards AM, Pai EF J Biol Chem. 2001 Mar 9;276(10):7225-32. Epub 2000 Nov 3. PMID:11063748[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||