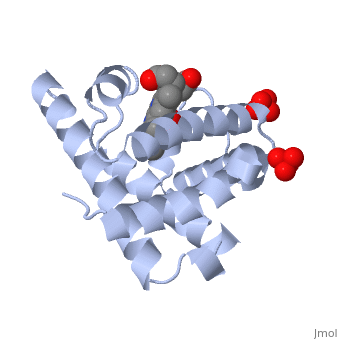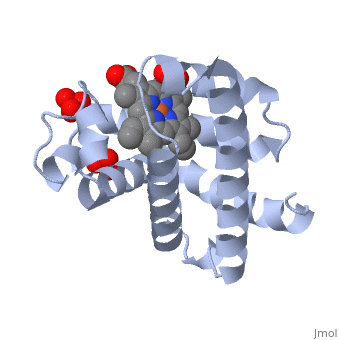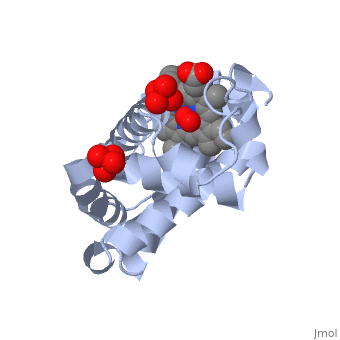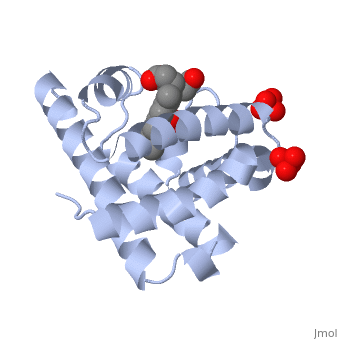Myoglobin: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
[[Myoglobin]] is a | [[Myoglobin]] is a protein found in muscle tissues that plays a critical role in the storage and transport of oxygen. It is structurally and functionally related to hemoglobin, the protein responsible for oxygen transport in red blood cells. While hemoglobin carries oxygen throughout the body, myoglobin is primarily found in muscle cells and facilitates the uptake and release of oxygen within the muscles themselves. | ||
== Structural highlights == | == Structural highlights == | ||
The globin consists mostly of [[Helices in Proteins|alpha helices]] shown in <scene name='23/238129/2ndary_structure/2'>pink</scene>; it has no beta sheets and its non-helical segments mostly serve as links that connect the helices. Look down the barrel of some of the longer helices. Are they all straight? The eight structurally conserved alpha helices are labelled <scene name='23/238129/Helix_labels/2'>A through H</scene>. The protein is colored as a N-->C rainbow in this view; the N terminus is blue, while the C terminus is red. | Myoglobin is a globular protein whose function is to store molecular oxygen in muscles (myo = muscles)<ref>PMID:15339940</ref>. It has two main components: a single polypeptide chain, and heme ligand. The heme ligand is only <scene name='23/238129/Transparent_spacefill/2'>partially exposed</scene> to the surface; the majority of it is buried inside the protein. The overall <scene name='23/238129/Surface/1'>shape</scene> of myoglobin is approximately disc-shaped with a diameter that is about twice its thickness. The overall fold of the protein is conserved, especially the <scene name='23/238129/Hydrophobic/1'>hydrophobic</scene> core of the protein (shown in purple), but the sequence is more <scene name='23/238129/Conserved_cartoon/1'>variable</scene> on the surface. {{Template:ColorKey_ConSurf_NoYellow_NoGray}} '''Metmyoglobin''' (MMb) is the oxidized form of myoglobin.The globin consists mostly of [[Helices in Proteins|alpha helices]] shown in <scene name='23/238129/2ndary_structure/2'>pink</scene>; it has no beta sheets and its non-helical segments mostly serve as links that connect the helices. Look down the barrel of some of the longer helices. Are they all straight? The eight structurally conserved alpha helices are labelled <scene name='23/238129/Helix_labels/2'>A through H</scene>. The protein is colored as a N-->C rainbow in this view; the N terminus is blue, while the C terminus is red. | ||
The <scene name='23/238129/Heme/2'>heme ligand</scene>, and specifically the iron atom in the middle of the heme, is what binds oxygen in myoglobin. In this representation, the heme alone is shown in ball and stick form with its C, N and O atoms displayed as grey, blue, and red balls respectively. The iron atom is shown in orange, and is in spacefilling mode to better illustrate its interactions with the heme. The iron is bound by four nitrogen atoms found in the heme ring, as well as an <scene name='23/238129/Fe_ligands/1'>amino acid</scene> from the protein chain. Which amino acid from the myoglobin protein binds to the iron? Notice that in the oxygenated state, the iron is in the plane of the heme ring. In the <scene name='23/238129/Deoxy_heme_fe_plane/1'>deoxy</scene> (no oxygen) state, the Fe atom is slightly above the plane of the heme, and a second <scene name='23/238129/Deoxy_heme_his/1'>amino acid</scene> coordinates with the iron in the heme ring. | The <scene name='23/238129/Heme/2'>heme ligand</scene>, and specifically the iron atom in the middle of the heme, is what binds oxygen in myoglobin. In this representation, the heme alone is shown in ball and stick form with its C, N and O atoms displayed as grey, blue, and red balls respectively. The iron atom is shown in orange, and is in spacefilling mode to better illustrate its interactions with the heme. The iron is bound by four nitrogen atoms found in the heme ring, as well as an <scene name='23/238129/Fe_ligands/1'>amino acid</scene> from the protein chain. Which amino acid from the myoglobin protein binds to the iron? Notice that in the oxygenated state, the iron is in the plane of the heme ring. In the <scene name='23/238129/Deoxy_heme_fe_plane/1'>deoxy</scene> (no oxygen) state, the Fe atom is slightly above the plane of the heme, and a second <scene name='23/238129/Deoxy_heme_his/1'>amino acid</scene> coordinates with the iron in the heme ring. | ||
Revision as of 09:42, 11 June 2023
FunctionMyoglobin is a protein found in muscle tissues that plays a critical role in the storage and transport of oxygen. It is structurally and functionally related to hemoglobin, the protein responsible for oxygen transport in red blood cells. While hemoglobin carries oxygen throughout the body, myoglobin is primarily found in muscle cells and facilitates the uptake and release of oxygen within the muscles themselves. Structural highlightsMyoglobin is a globular protein whose function is to store molecular oxygen in muscles (myo = muscles)[1]. It has two main components: a single polypeptide chain, and heme ligand. The heme ligand is only to the surface; the majority of it is buried inside the protein. The overall of myoglobin is approximately disc-shaped with a diameter that is about twice its thickness. The overall fold of the protein is conserved, especially the core of the protein (shown in purple), but the sequence is more on the surface. The , and specifically the iron atom in the middle of the heme, is what binds oxygen in myoglobin. In this representation, the heme alone is shown in ball and stick form with its C, N and O atoms displayed as grey, blue, and red balls respectively. The iron atom is shown in orange, and is in spacefilling mode to better illustrate its interactions with the heme. The iron is bound by four nitrogen atoms found in the heme ring, as well as an from the protein chain. Which amino acid from the myoglobin protein binds to the iron? Notice that in the oxygenated state, the iron is in the plane of the heme ring. In the (no oxygen) state, the Fe atom is slightly above the plane of the heme, and a second coordinates with the iron in the heme ring. Additional detailsOxymyoglobin for myoglobin complex with O2 Porphyrin for porphyrin.
Myoglobin-Physeter-catodon-structure (Spanish) 3D Structures of Myoglobin
|
| ||||||||||
External ResourcesExternal Resources
ReferencesReferences
- ↑ Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol. 2004 Sep;207(Pt 20):3441-6. PMID:15339940 doi:http://dx.doi.org/10.1242/jeb.01172