Gluconeogenesis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 31: | Line 31: | ||
'''Fatty acids''' | '''Fatty acids''' | ||
Odd-chain fatty acids can be oxidized to yield <scene name='43/430893/Cv/2'>Acetyl-CoA</scene> and <scene name='92/925544/Cv/4'>Propionyl-CoA</scene>, the latter serving as a precursor to <scene name='43/430893/Cv/9'>Succinyl-CoA</scene>, which can be converted to <scene name='39/392339/Cv1/11'>pyruvate</scene> and enter into gluconeogenesis. In contrast, even-chain fatty acids are oxidized to yield only acetyl-CoA, whose entry into gluconeogenesis requires the presence of a glyoxylate cycle (also known as glyoxylate shunt) to produce four-carbon dicarboxylic acid precursors. | Odd-chain fatty acids can be oxidized to yield <scene name='43/430893/Cv/2'>Acetyl-CoA</scene> and <scene name='92/925544/Cv/4'>Propionyl-CoA</scene>, the latter serving as a precursor to <scene name='43/430893/Cv/9'>Succinyl-CoA</scene>, which can be converted to <scene name='39/392339/Cv1/11'>pyruvate</scene> and enter into gluconeogenesis. In contrast, even-chain fatty acids are oxidized to yield only acetyl-CoA, whose entry into gluconeogenesis requires the presence of a [[glyoxylate cycle]] (also known as glyoxylate shunt) to produce four-carbon dicarboxylic acid precursors. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 18:07, 28 November 2022
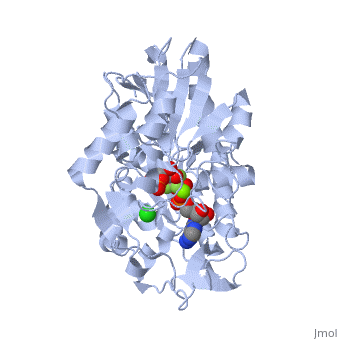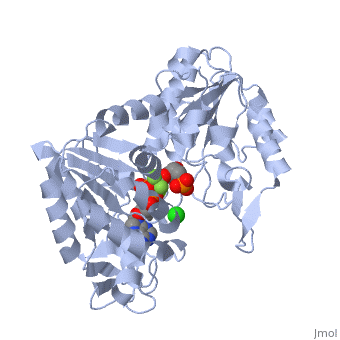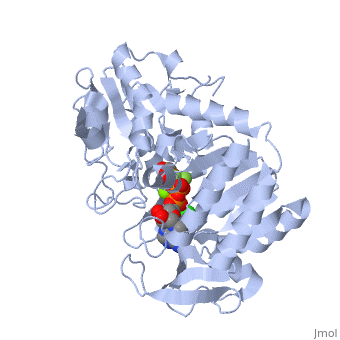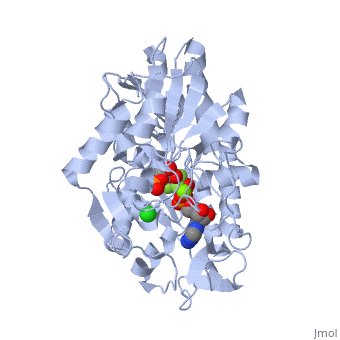
Under development! Gluconeogenesis is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. In humans the main gluconeogenic precursors are lactate, (which is a part of the triglyceride molecule), alanine and glutamine. Other glucogenic amino acids and all Citric Acid Cycle intermediates (through conversion to oxaloacetate) can also function as substrates for gluconeogenesis. is transported back to the liver where it is converted into by the Cori cycle using the enzyme lactate dehydrogenase. . Pyruvate, the first designated substrate of the gluconeogenic pathway, can then be used to generate glucose. Phosphoglycerate kinase is an enzyme that catalyzes the reversible transfer of a phosphate group from to ADP producing and ATP: 1,3-bisphosphoglycerate + ADP ⇌ glycerate 3-phosphate + ATP Like all kinases it is a transferase. PGK is a major enzyme used in glycolysis, in the first ATP-generating step of the glycolytic pathway. In gluconeogenesis, the reaction catalyzed by PGK proceeds in the opposite direction, generating ADP and 3-phosphoglycerate (3-PG). Catabolism of amino acids, Citric Acid Cycle and Gluconeogenesis 1) , cysteine, glycine, serine, tryptophan, threonine => 2) Tryptophan, threonine, phenylalanine, tyrosine, isoleucine, leucine, lysine => 3) Arginine, histidine, glutamine, proline => glutamate => 4) Threonine, methionine, isoleucine, valine => 4C chain (CoA excluded) 5) Tyrosine, phenylalanine, aspartate => 6) Aspartate, asparagine => => [by Phosphoenolpyruvate carboxykinase] (PEP) => ... => The location of the enzyme that links these two parts of gluconeogenesis by converting oxaloacetate to PEP – PEP carboxykinase (PEPCK) – is variable by species: it can be found entirely within the mitochondria, entirely within the cytosol, or dispersed evenly between the two, as it is in humans. E. coli GTP-driven PEPCK is located in a pocket at the enzyme surface[1]. Water molecules are shown as red spheres. Fatty acids Odd-chain fatty acids can be oxidized to yield and , the latter serving as a precursor to , which can be converted to and enter into gluconeogenesis. In contrast, even-chain fatty acids are oxidized to yield only acetyl-CoA, whose entry into gluconeogenesis requires the presence of a glyoxylate cycle (also known as glyoxylate shunt) to produce four-carbon dicarboxylic acid precursors.
|
| ||||||||||
ReferencesReferences
- ↑ Dunten P, Belunis C, Crowther R, Hollfelder K, Kammlott U, Levin W, Michel H, Ramsey GB, Swain A, Weber D, Wertheimer SJ. Crystal structure of human cytosolic phosphoenolpyruvate carboxykinase reveals a new GTP-binding site. J Mol Biol. 2002 Feb 15;316(2):257-64. PMID:11851336 doi:http://dx.doi.org/10.1006/jmbi.2001.5364