Methionine synthase: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
1K7Y - cobalamin | 1K7Y - cobalamin | ||
1K98 - AdoMet complex | 1K98 - AdoMet complex | ||
<scene name='90/907471/Superposition_1/2'>Methionine synthase domains</scene> | |||
</StructureSection> | </StructureSection> | ||
| Line 17: | Line 19: | ||
[[Image:Overall.jpeg]] | [[Image:Overall.jpeg]] | ||
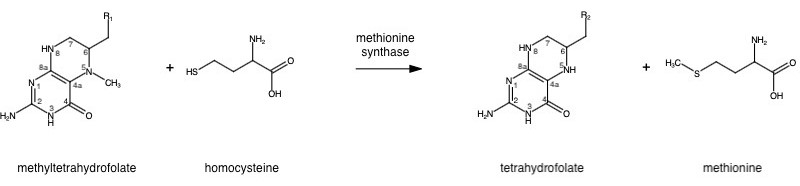
The change from homocysteine to methionine is an SN2 reaction where the methyl group from methyltetrahydrofolate (MTHF), located on N-5, is donated. MTHF is a product of MTHFR. | The change from homocysteine to methionine is an SN2 reaction where the methyl group from methyltetrahydrofolate (MTHF), located on N-5, is donated. MTHF is a product of MTHFR. | ||
Revision as of 20:57, 5 April 2022
Methionine synthaseMethionine synthase
This page is being worked on during the Spring 2022 semester. Methionine is an amino acid our bodies require to ensure normal healthy tissue growth and repair. It is not made naturally in the body and can only be obtained from our diets first in the form of homocysteine. A lack of or deficiencies of methionine has been linked to diseases such as poor growth and birth abnormalities[1]. Homocysteine, another amino acid most commonly found int he liver, is converted to methionine. EC: 2.1.1.13 PDB ID: 1K7Y - cobalamin 1K98 - AdoMet complex
|
| ||||||||||
The change from homocysteine to methionine is an SN2 reaction where the methyl group from methyltetrahydrofolate (MTHF), located on N-5, is donated. MTHF is a product of MTHFR.
This is a complex reaction as the product, tetrahydrofolate, is a poor leaving group, thus requiring a "supernucleophile" with a protein-bound B-12 vitamin Cobalamin as the methyl carrier.
Vitamin B-12Vitamin B-12
Oxidation States of CobalaminOxidation States of Cobalamin
RelevanceRelevance
Structural highlightsStructural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
ReferencesReferences
- ↑ Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06